Cancer ranks among the leading causes of death worldwide, claiming approximately 10 million lives in 2022, according to GLOBOCAN data (Bray et al., 2024). This disease is characterized by the uncontrolled proliferation of abnormal cells, their evasion of cellular checkpoints that generally regulate cell growth and death, and their invasion into surrounding tissues (Hanahan, 2022). The complexity and adaptability of cancer cells make the disease particularly challenging to treat. To date, chemotherapy is one of the most prevalent and effective methods in addition to surgery for the treatment of patients (Anand et al., 2023). Despite significant advancements in the development of chemotherapeutic agents, resistance to these treatments remains a major challenge. Chemoresistance has been observed for nearly all chemotherapeutic agents, including commonly used drugs such as doxorubicin, paclitaxel, 5-fluorouracil (5-FU), cisplatin, and gemcitabine (Mollaei et al., 2021). This chemoresistance is multifaceted and can be attributed to several factors, with deficiencies in programmed cell death (PCD) being one of them (Sazonova et al., 2024).
CD44, a cell surface transmembrane glycoprotein, is known to regulate tumor progression and cancer-associated molecular signaling pathways. Notably, CD44 variant exon-containing isoforms, absent in norma and appearing during tumorigenesis due to alternative splicing, can interact with various cell membrane receptors/intracellular proteins, influencing signaling that promotes cell survival. A recent review indicates that CD44 (including CD44 variant exon containing isoforms) is involved in MAPK, Hippo and PI3K/Akt signaling pathways to name a few (Xu et al., 2024). Cell survival signaling is tightly interconnected with the sensitivity of cells to chemotherapeutic drugs. Consequently, through the regulation of cell death signaling pathways, CD44 variant exon containing isoforms may be presented as targets for selective modulation of chemosensitivity. However, how particular CD44 exons are involved in cell death pathways modulation is still under question. Hence, this review explores the association of CD44 variant exon expression with clinical outcomes of patients undergoing chemotherapy, highlights their role in key cell death pathways regulation, provides critical analysis of therapeutic substances and clinical studies targeting CD44 and discusses directions for further development of CD44 targeted therapeutic strategies.
Importantly, among the nine variant exons of CD44 (v2-v10), our review focuses on three exons—v3, v6, and v9—that are associated with patient survival following chemotherapy. The molecular mechanisms underlying these associations have been described in relation to their biological function both in vitro and in vivo (with the exception of v3). Although the expression levels of variant exons v7 and v10 have been shown to correlate with patient survival, the existing literature on the molecular mechanisms of chemoresistance for these exons is limited. As a result, we do not cover them in this review.
2 Molecular structure and functional Basis of CD44 and its splicing variantsCD44, a non-kinase cell surface transmembrane glycoprotein, was described in 1989 by Stamenkovic et al., and two main forms of the protein were identified–a lymphoid form present in hematopoietic cells and an epithelial form weakly expressed in normal epithelium and abundantly expressed in carcinomas (Stamenkovic et al., 1989). CD44 activity is modulated by interaction with its ligands, mainly with hyaluronan (HA) (Aruffo et al., 1990).
Research has identified that CD44 is encoded by a highly conserved gene on the short arm of human chromosome 11 (Screaton et al., 1992). Nineteen exons are involved in the genomic organization of this molecule. As a result of alternative splicing and posttranslational modifications, at least 21 predicted CD44 isoforms are generated with 8 experimentally confirmed isoforms with different molecular weight (in the range of 85–250 kDa) and function (Gaiteiro et al., 2022). The smallest isoform, alternatively named hematopoietic or standard isoform 4 (CD44s, isoform 4), is ubiquitously expressed in vertebrate cells of mesenchymal origin, including leukocytes, fibroblasts and neuronal cells (Chen et al., 2020; Naor et al., 2008) and is translated from the first five (exons 1-5 or s1-5) and the last four (exons 15–17 and 19 or s15–17 and s19) exons (Figure 1). On the contrary, nine variant exons (exons 6–14 or v2-v10), located in the middle of the CD44 gene can be alternatively spliced and assembled with exons contained in CD44s, resulting in the formation of a plethora of CD44 variant isoforms, typical of cells of epithelial origin and several carcinomas. The mechanisms by which variant isoforms of CD44 are formed by alternative splicing are described in detail in the recent review (Maltseva and Tonevitsky, 2023).
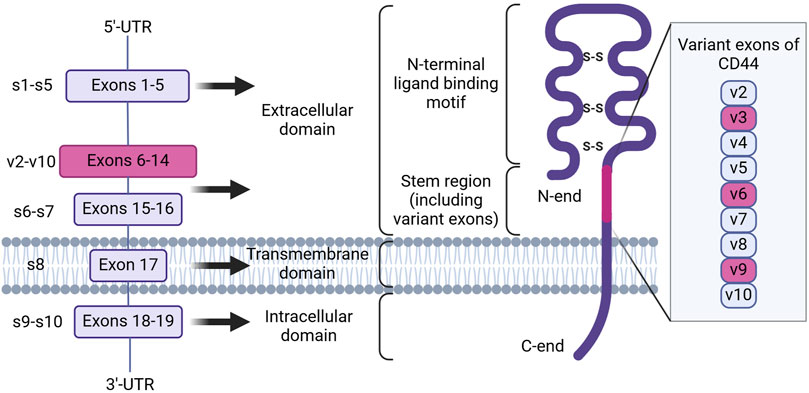
Figure 1. The genomic organization and protein structure of human CD44. CD44 human gene contains 19 exons. Alternative splicing results in the formation of CD44 standard and variant isoforms. CD44 protein consists of an intracellular and transmembrane domain, consisting of exons 18–19 and 17, and an extracellular domain, consisting of exons 1–16. Inclusion of variant exons (pink) to CD44 isoforms is observed during cancer progression and chemoresistance, with the latter being associated with variant exons v3, v6 and v9 (Created with Biorender.com).
At present, all CD44 isoforms belong to the type 1 transmembrane protein class and have a typical structure consisting of the extracellular domain (ECD), the transmembrane domain (TMD) and the cytoplasmic or intracellular domain (ICD) (Figure 1; Chen et al., 2020). The ECD, in turn, includes several regions necessary for sensoring, structural integrity and activation of CD44 protein. Firstly, the N-terminal ligand-binding motif or HA-binding domain (HABD), oriented into the extracellular matrix, is present in all isoforms of CD44 (both CD44s and CD44 variant isoforms), consists of constant exons 1-5 and forms a compactly-folded domain. HABD allows interaction with extracellular matrix components such as HA, osteopontin, fibronectin, collagen, growth factors, cytokines and matrix metalloproteinases in a glycosylation- and disulfide-bond reduction-dependent manner (Senbanjo and Chellaiah, 2017). Secondly, the variant region present in variant isoforms can vary significantly in length depending on what exons (6–14) are included in the mRNA after splicing (resulting, for example, in the formation of isoforms CD44v2-v10, CD44v3-v10, CD44v8-v10 or an isoform containing only one CD44v10 variant exon) and forms additional sites of interaction with growth factors and receptors on the plasma membrane, consequently expanding the functionality of CD44 (Liao et al., 2022). Lastly, the stem region is made up of exons 15 and 16. The TMD consists of exon 17 and contains a cysteine residue responsible for palmitoylation and consequent incorporation into lipid rafts of the cellular membrane. It also has been shown to provide a platform for CD44 oligomerization and coupling to adaptor proteins, receptor tyrosine kinases (RTKs) or nonreceptor protein-tyrosine kinases for consequent CD44 activation and signaling (Sun et al., 2020). The ICD contains a nuclear localization signal (NLS) for outside-in-cell signaling, consisting of two clusters of basic amino acids (292RRRCGQKKK300) (Skandalis, 2023). CD44 ICD can be cleaved from the membrane by presenilin-γ-secretase and transported into the nucleus, where it can act as a transcription factor through binding to consensus sequences found in the promoter regions of genes, including CD44 itself.
3 The expression profile of CD44 splice variants in cancerInsight on the expression profile of different splice variants of CD44 can be of use for better understanding of cancer-specific molecular fingerprints and choosing an optimal cancer targeting strategy. In this section we compile what is known on the CD44 isoforms/exons expression profile from tumor samples and cell lines of clinically relevant cancers, as well as conduct an independent analysis using The Cancer Genome Atlas (TCGA) RNA sequencing (RNA-seq) data on CD44 splice isoforms derived from the GEPIA2 database (Figure 2; Tang et al., 2019). It should be noted that apart from experimentally determined CD44 isoforms in NCBI and UniProt databases used in our analysis, additional predicted CD44 isoforms exist (Supplementary Figure S1; Maltseva and Tonevitsky, 2023; Tang et al., 2019; Shi et al., 2024).
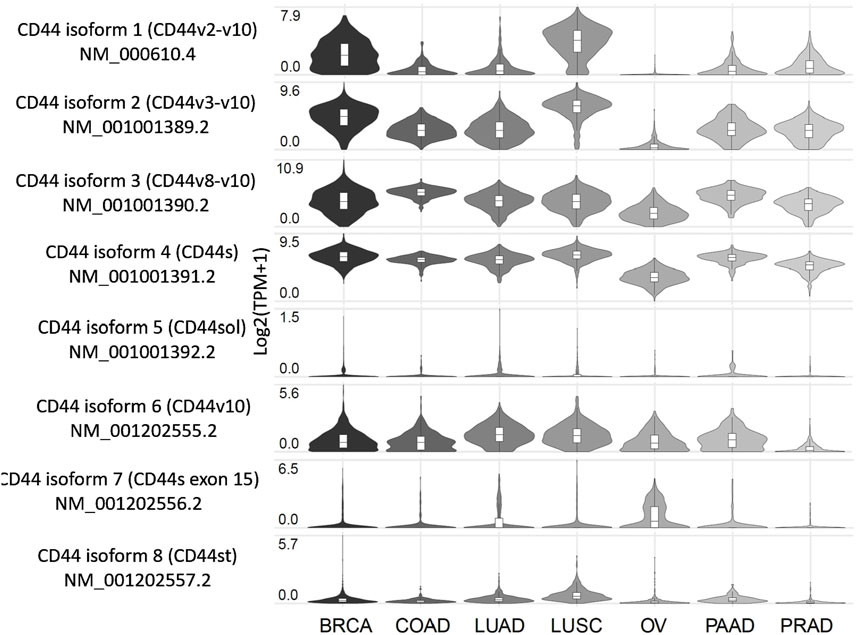
Figure 2. Expression profile of CD44 splice variants in patients with several tumor types, including the most common and those associated with the highest mortality. Created by www.gepia2.com.
Below, we examine the results for several of the most common tumor types and those associated with the highest mortality rates (results for other tumor types can be found in Supplementary Figure S1). According to the GEPIA2 database (Figure 2), breast cancer (BRCA) patients predominantly express CD44 isoform 4 (CD44s), along with isoforms 1-3, which include exons v2-v10, and to a lesser extent, isoform 6 (CD44v10). Squamous cell lung cancer (LUSC) patients exhibit a similar expression profile to that of BRCA. Patients with colorectal (COAD), lung (LUAD), pancreatic (PAAD) and prostate (PRAD) cancers primarily express CD44s as well as isoforms 2 and 3, which include exons v3-v10. Notably, LUAD and PAAD patients also express isoform 6, which includes exon v10. Ovarian cancer patients (OV) tend to express CD44s and isoform 3 (CD44v8-v10), although it is worth noting that the expression levels are lower compared to those observed previously mentioned cancers.
According to literature, BRCA patients in accordance with TCGA data express isoforms 1–4 (Bànkfalvi et al., 1998; Tokue et al., 1998; Olsson et al., 2011; Table 1). Interestingly, in grade 1 invasive ductal carcinoma patients, more than 50% of tumor samples stain positive for CD44 variant exons v3, v5-v7 and v9 (CD44v3, CD44v5, CD44v6, CD44v7 and CD44v9), highlighting their potential as therapeutic targets for early grade ductal carcinoma (Bànkfalvi et al., 1998). However, as ductal carcinoma progresses to grades 2–3, an increase in tumor samples staining positive for CD44s and CD44v9 is observed, along with a decrease in samples staining positive for CD44v3, CD44v6 and CD44v7. This shift suggests that targeting CD44 variant exons becomes less feasible as the tumor advances (Bànkfalvi et al., 1998). Additionally, another study points to more than 50% of tumor samples stain positive for CD44v6, supporting its relevance (Tokue et al., 1998).
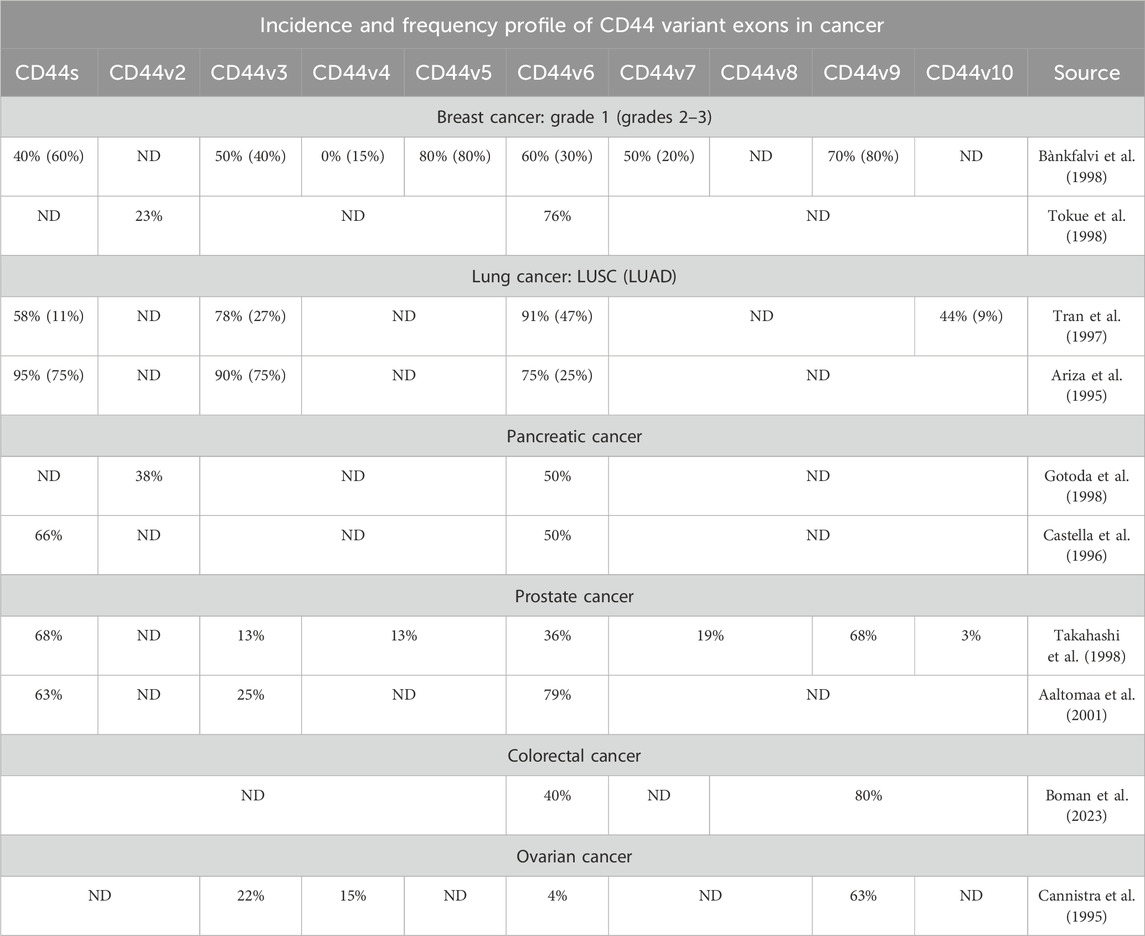
Table 1. The incidence and frequency profile of CD44 variant exons expression in several tumor types, including the most common and those associated with the highest mortality.
Several studies on non-small cell lung cancer (NSCLC) have identified CD44v3, CD44v6 and CD44v10 in more than half of the patients with LUSC, whereas only CD44v6 and to a lesser extent CD44v3 are expressed in patients with LUAD (Tran et al., 1997; Table 1). These findings suggest that LUSC could potentially be targeted via several CD44 variant exons, while CD44 targeting in LUAD CD44 is predominantly restricted to CD44v3 (Tran et al., 1997). Additionally, in PAAD and PRAD patients, CD44v6 is expressed prominently. In PAAD, CD44v2 and CD44v3 are expressed to a lesser extent, whereas in PRAD, CD44v6 and CD44v9 are expressed. Notably, more than half of PRAD patients show positivity for CD44v6 and CD44v9 (Gotoda et al., 1998; Castella et al., 1996; Takahashi et al., 1998; Aaltomaa et al., 2001).
Research concerning CD44 splice variants expression profile has been conducted on TCGA patients and on colorectal cancer cell lines using TCGA RNA-seq data (Novosad et al., 2022; Everest-Dass et al., 2024). It was identified that CD44 isoforms 2, 3 and 4 are predominantly expressed in colorectal cancer patients at the mRNA expression levels (Novosad et al., 2022). Moreover, CD44v6 and CD44v8-v10 were shown to be positively expressed in approximately more than half of the colorectal cancer tumors (Boman et al., 2023; Table 1).
In ovarian cancer, only CD44v9 was shown to be identified in more than half of the patient tumors (Cannistra et al., 1995). This is in accordance with TCGA data, since OVCA patients express only CD44s and CD44 isoform 3.
Overall, the cancers analyzed exhibit distinct expression profiles of CD44 isoforms/variant exons, based on our findings and immunohistochemical data available in the literature. According to TCGA data (GEPIA2), three distinct groups can be characterized by their CD44 isoform expression pattern: 1) cancers expressing CD44 isoforms 1-4 and consequently variant exons v2-v10, 2) cancers expressing CD44 isoforms 2-4 and variant exons v3-v10, and 3) cancers expressing only CD44 isoforms 3–4 (variant exons v8-v10). Moreover, the results of immunohistochemical analyses of expression profiles of CD44 isoforms/variant exons in tumor samples closely align with TCGA data. Differences in the CD44 isoform/variant exon expression profiles underscore the need for tailored CD44-targeting approaches.
4 Chemotherapy and cell death pathways modulationCell death, a crucial aspect of the response to chemotherapy, can occur through various mechanisms, each influencing chemoresistance: during tumor progression, the balance between pro-cell death and anti-cell death regulators is shifted towards survival by various escape mechanisms (D’Amico and De Amicis, 2024). Recent advances in research have significantly expanded our understanding of cell death processes, introducing several new mechanisms. The Nomenclature Committee on Cell Death (NCCD) has updated its guidelines to include these novel forms of cell death, reflecting the increasing complexity of the field (Galluzzi et al., 2018). Apart from well-known apoptosis and autophagy, types of cell death, such as ferroptosis, an iron-dependent form of cell death characterized by the accumulation of lipid peroxides, were included (Dixon and Olzmann, 2024).
Apoptosis, discovered in 1972, is a programmed and controlled process of cell self-destruction requiring specific cell signaling transduction (Wang et al., 2023). The use of anticancer drugs to induce cell death by apoptosis has been considered one of the most critical ways to kill cancer cells, partly due to the fact that it has been considered the only form of cell death. Many chemotherapy drugs are known to induce cellular death through apoptosis, including cisplatin, 5-FU and gemcitabine (Kaufmann and Earnshaw, 2000).
Ferroptosis is a nonapoptotic iron-dependent form of cell death driven by lipid peroxidation (Dixon et al., 2012). Ferroptosis has recently been proven to correlate with cancer therapy resistance. A plethora of studies have identified that regulation of ferroptosis could influence the efficacy of cancer treatment and reverse cancer therapy resistance (Friedmann Angeli et al., 2019; Wu et al., 2020). Some chemotherapy drugs have been found to induce ferroptosis, including cisplatin and gemcitabine (Zhou et al., 2024).
Autophagy is a biological process that allows cells to degrade and recycle proteins and organelles to maintain homeostasis and adapt to various stresses (Liu et al., 2023). Chemotherapeutic agents such as paclitaxel, docetaxel, cisplatin, doxorubicin and 5-FU are known to induce autophagy (Debnath et al., 2023). Moreover, research has shown that the stimulation of autophagy by paclitaxel and cisplatin prevents apoptosis of tumor cells and leads to cancer chemoresistance (Debnath et al., 2023; Li et al., 2021).
Overall, chemotherapy drugs commonly utilized in clinic have been shown to induce not only apoptosis, but additionally ferroptosis and autophagy. This may be useful in the sense that resistance to apoptosis is a widespread noted effect, and cells that are resistant to apoptosis remain sensitive to ferroptosis (dos Santos et al., 2023). Therefore, the combinational or seldom triggering of different cell death pathways may be helpful for chemoresistance alleviation.
According to a recent scoping review of clinical studies, the impact of CD44 expression on the efficacy of chemotherapy treatment primarily correlates with drugs such as 5-FU, cisplatin and docetaxel (Wu et al., 2024). These drugs are widely used to treat locally advanced or metastatic breast, colorectal, head and neck cancer, among others. However, how particular CD44 exons are involved in cell death pathways modulation is still under question. Hence, in our review, we focus on the effect exerted by variant exons that are alternative to independent isoforms since several isoforms structurally share the same exons and, therefore, could participate in similar exerted processes.
5 Total CD44 and chemotherapy resistance5.1 Effect of total CD44 expression on chemotherapy treatment outcome/tumor response to chemotherapy in patients/animal studiesFirstly, the current section of our review will focus on total CD44 involvement in chemoresistance, since total CD44-mediated signaling may involve both standard and/or variant exon containing isoforms. Onwards, specific variant exons of CD44 will be discussed.
Overall, a significant negative effect of increased total CD44 expression on chemotherapy treatment outcome was observed across several types of cancers, including breast, colorectal and head and neck cancers measured typically through overall survival (OS) and recurrence-free survival (RFS) values, as well as through response to chemotherapy (Wu et al., 2024). Notably, several studies have identified no correlation or even a positive correlation with response to chemotherapy treatment outcome, which underlines the ambiguity of CD44’s relationship with chemotherapy outcomes (Wu et al., 2024). Such differences may be due to the variation of CD44 variant isoforms amongst different cancer types and the difference in activated survival pathways. Varying CD44 isoform/variant exon detection methods may also contribute to these differences.
In addition, the association between total CD44 overexpression/knockdown and chemoresistance in vivo has been studied (Table 2). Research indicates that CD44 knockdown (CD44kd) in prostate cancer xenografts significantly reduces tumor growth rate after continuous docetaxel treatment compared with control cells, suggesting that the combination of chemotherapy with CD44kd can lead to a beneficial chemotherapy outcome (Hao et al., 2012).
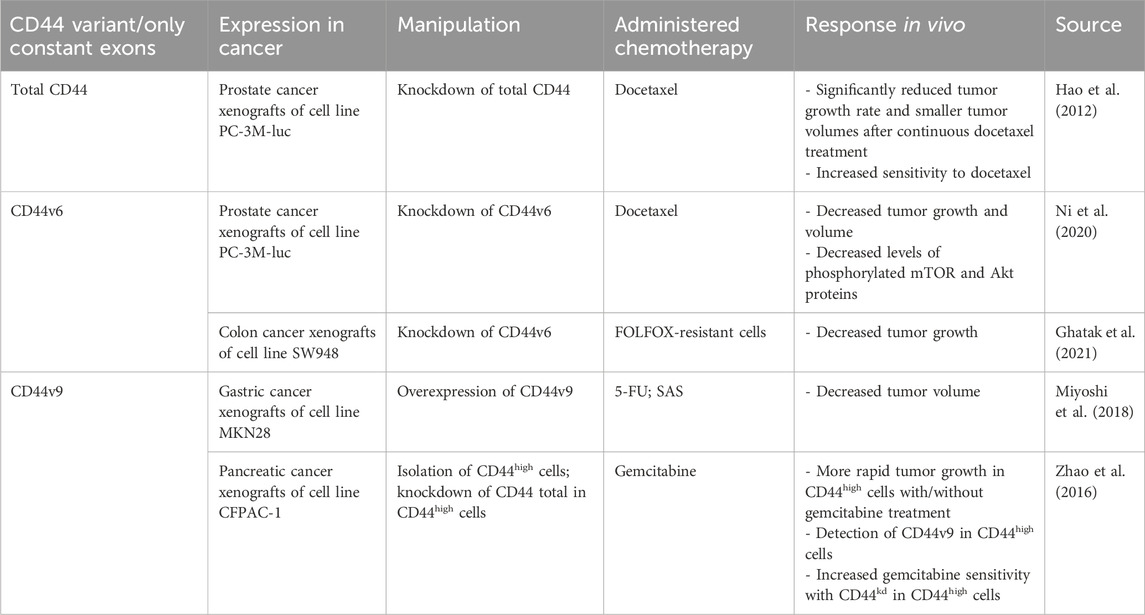
Table 2. The association of CD44 variant exon expression levels with tumor response to chemotherapy in animal studies.
Literature analysis demonstrated a predominantly negative relationship between total CD44 expression levels and the clinical outcome of patients. Moreover, CD44kd cancer xenografts in vivo significantly reduce tumor growth rate after continuous chemotherapy administration. Thus, total CD44, despite the presence of both standard and variant isoforms, seems to be involved in chemotherapy resistance.
5.2 Total CD44 biological functions in relation to cell death pathwaysThe underlying mechanisms of total CD44 in chemotherapy resistance are quite diverse, possibly due to its combination of several variant exon-containing isoforms and the standard isoform (Table 3). It has been shown to influence key cell death signaling pathways, including apoptosis, ferroptosis and autophagy.

Table 3. CD44 variant exon biological functions in relation to cell death pathways.
It was shown that CD44 is able to form a complex with receptor tyrosine-protein kinases 2 (ERBB2) (Ghatak et al., 2005). For instance, HA oligomers, inhibitors of HA interaction with CD44, were shown to prevent the assembly of phosphorylated ERBB2, CD44, ezrin, Hsp90/Cdc37 and p110a into a complex, which is then able to exert ERBB2 associated PI3K/Akt signaling pathway, promoting apoptosis resistance (Ghatak et al., 2005; Figure 3A). Such an interaction demonstrates the involvement of CD44 in growth factor receptor signaling promotion.
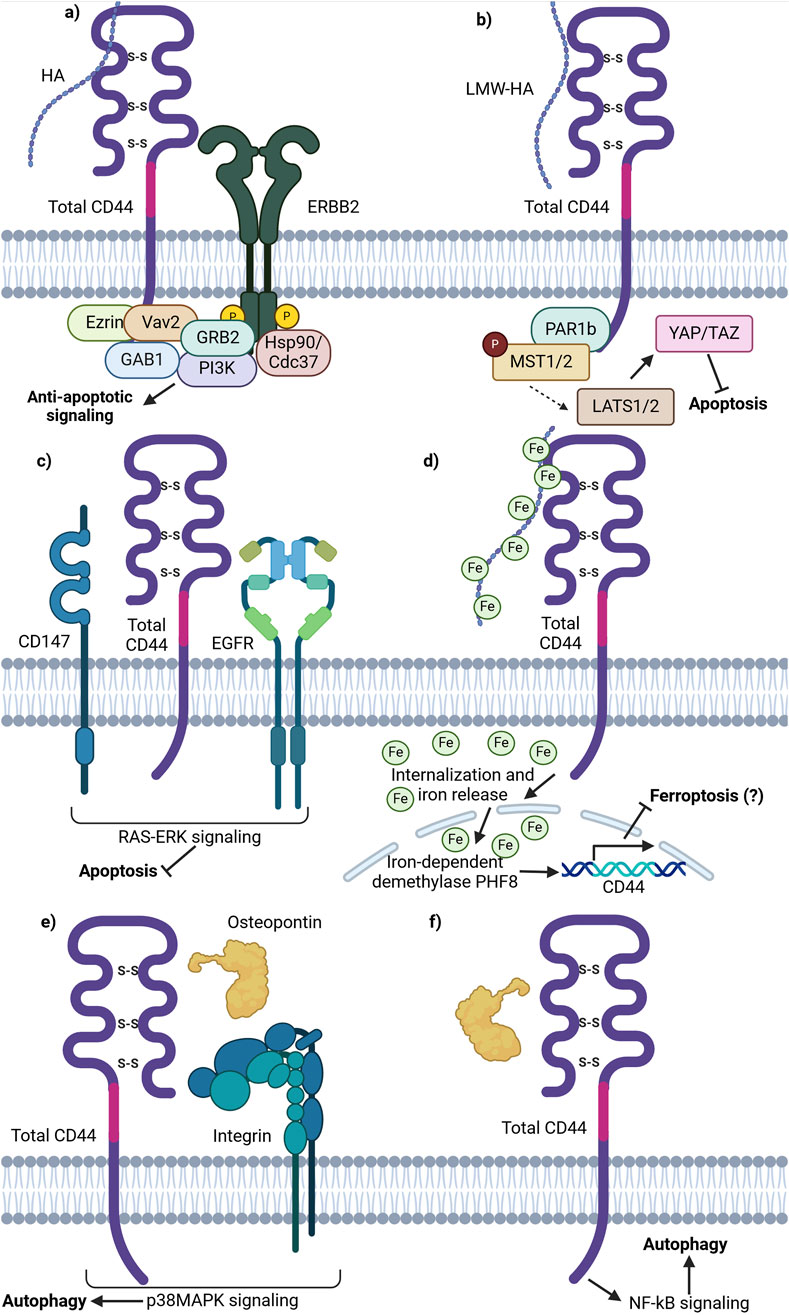
Figure 3. Schematic representation of total CD44 (CD44v and CD44s) involvement in cell death pathways. (A) CD44 is able to form an anti-apoptotic complex with ERBB2. CD44 binds ezrin, Vav2 and GAB1 at the ICD. ERBB2 recruits GRB2, Hsp70/Cdc37 and PI3K. The complex formation results in PI3K/Akt anti-apoptotic signaling promotion upon HA stimulation. (B) Binding of LMW-HA by CD44 leads to inhibitory phosphorylation of MST1/2 by PAR1b and consequent YAP activation, thereby promoting apoptosis inhibition and cell growth and proliferation activation. (C) Lipid raft associated CD44, CD147 and EGFR complex promotes EGFR signaling through the RAS-Erk downstream signaling proteins, conferring anti-apoptotic signaling. (D) Total CD44 is involved in the uptake of HA-bound iron, necessary for the function of iron-dependent proteins including nuclear demethylases. Nuclear demethylase PHF8, activated upon iron binding, promotes the expression of CD44 in a positive-feedback loop. (E) OPN-CD44-ITG-p38MAPK promotes autophagy. OPN-induced autophagy was inhibited by adding an ITG inhibitor RGD and/or anti-CD44 antibody; p38MAPK inhibitor SB203580 significantly attenuated autophagy by decreasing the expression levels of ATG4B, BECLIN1/ATG6, BNIP3 and VPS34 (not shown). (F) OPN-CD44-NF-κB promotes autophagy. NF-κB inhibitor BAY 1170–82 significantly inhibited the OPN-induced LC3-II expression and cell populations with CD44+/CD133+ expression profile (Created with BioRender.com).
Moreover, research on CD44 influence on cell death signaling pathways uncovered its involvement in the Hippo pathway. It was demonstrated that upon HA binding to CD44, CD44 clustering on the cellular membrane is initiated, stimulating the interaction of its intracellular domain with polarity-regulating kinase (PAR1b) (Ooki et al., 2019). Notably, it has been demonstrated that in normal tissues with increasing cell densities, CD44 is activated by high molecular weight (HMW)-HA, thereby initiating PAR1b phosphorylation process of mammalian STE20 like kinase 1 and 2 (MST1/2) and activation of Yes associated protein (YAP). Such a process results in the inhibition of cell growth. Similarly, merlin/neurofibromin-2 (Mer/NF2) also acts as a downstream effector, transferring signals from HA-CD44 activation. CD44 activation by HMW-HA reduces Mer phosphorylation, leading to its activation. Activated Mer has been shown to inhibit PI3K/Akt signaling (Ooki and Hatakeyama, 2020). Overall, in parallel with PAR1b, Mer acts as downstream effector molecules of the HA/CD44/Hippo pathway, modulating its activity. Since the Hippo pathway is an essential survival-associated signaling pathway, its’ inactivation promotes cell proliferation and decreases apoptosis, contributing to tumor initiation, progression and chemoresistance (Figure 3B).
Another pathway utilized by tumor cells to prevent apoptosis is signaling through the CD44/CD147/EGFR complex. On one hand, it was demonstrated that the knockdown of total CD44 leads to decreased EGFR activity levels, pointing to their functional linkage with one another (Grass et al., 2013). On the other hand, CD147 was shown to promote HA synthesis, thereby acting on CD44 activity levels through ligand interactions (Grass et al., 2013). The authors further demonstrated that the CD44/CD147/EGFR complex forms and promotes ERK activation through enhanced CD44-HA signaling in lipid rafts (Grass et al., 2013). While the impact of ERK activation on apoptosis was not directly analyzed, EGFR-Ras-ERK signaling is known to inhibit apoptosis and promote cell survival, thereby implying yet another case of CD44 apoptosis regulation through the interaction with cell membrane receptors (Figure 3C; Ullah et al., 2022).
Apart from apoptosis resistance, total CD44 may be involved in ferroptosis resistance. Müller et al. described a novel mechanism involving hyaluronan-bound iron transfer into the cell via CD44, influencing gene expression regulation (Müller et al., 2020; Figure 3D). The authors demonstrated that LMW-HA bound by iron is internalized intracellularly in complex with CD44 by endocytosis. Similar to transferrin-bound iron, CD44-HA-bound iron is further released into the cytoplasm upon acidification of the endosome and can then be transferred into specific cellular compartments. In particular, CD44-HA-bound iron increases the nuclear iron pool and reduces the histone mark H3K9me2 in an iron-dependent manner (Müller et al., 2020). This leads to the upregulation of genes, including CD44 itself. While no current studies suggest that CD44-mediated iron flux directly contributes to chemoresistance, a potential link may exist. Additionally, the impact of different CD44 isoforms on the CD44-HA-iron feedback loop remains unclear.
Total CD44 is additionally known to regulate autophagy in cancer cells. For instance, autophagy was shown to promote a CD44high/CD24low phenotype in breast cancer stem cells (CSCs) – such a cell expression profile was identified in a smaller portion of cells upon knockdown of several autophagy initiating proteins (ATG8/LC3B and ATG12) (Cufí et al., 2011). Moreover, the treatment of CD44high/CD24low cells with an autophagy inhibitor chloroquine (CQ) was shown to shift the cancer cell phenotype to the opposite (CD44low/CD24high) (Cufí et al., 2011). Additionally, the CD44 ligand osteopontin (OPN) has been implicated in autophagy induction. In vascular smooth muscle cells (SMCs), OPN was shown to trigger autophagosome formation (Kariya and Kariya, 2022). This process was inhibited by an integrin (ITG) inhibitor (RGD peptide) or anti-CD44 antibody, highlighting the importance of CD44 and RDG motif-containing integrins in autophagy initiation (Figure 3E). In another study, OPN activation was shown to stimulate NF-κB, ERK, and STAT3 signaling pathways (Figure 3F; Zheng et al., 2012). Thus, literature suggests that total CD44 and its ligands are linked with autophagy initiation and the stemness properties of tumor cells, thereby promoting resistance to administered therapy.
Overall, the mechanisms responsible for total CD44 mediation of cell death pathways are diverse. They include the influence of apoptosis resistance through ERBB2 complex, CD147 and CD44 interaction with LMW-HA. Currently, no research highlights that iron flux mediated by CD44 significantly contributes to chemoresistance, but there may be an association. Whether different CD44 isoforms alter their expression patterns within the CD44-HA bound iron positive feedback loop remains unclear. Further studies are required to clarify these possible associations and their relationship with chemoresistance. Lastly, total CD44 was shown to promote autophagy and chemoresistance in several cancers through OPN-CD44-ITG-p38MAPK and OPN-CD44-NF-κB signaling. Thus, targeting both cell death pathways and total CD44 signaling may offer a promising approach to overcoming chemoresistance in various cancers. However, it should be mentioned that additional research comparing the effect of standard and variant isoforms in mediating cell death signaling may be necessary for further precision in targeting chemoresistant cells.
5.3 Total CD44 therapeutic strategies and clinical implicationsSeveral approaches targeting total CD44 (including variant and standard isoforms) for chemotherapy resistance alleviation have been developed and analyzed in vivo (Table 4). Some approaches for CD44-exerted chemoresistance treatment entail using CD44-targeting gene therapies, delivery systems and cell therapies. Regarding gene therapy strategies, complementary DNA (cDNA) vaccine to CD44 variant isoforms, but not CD44s, demonstrated decreased tumor growth, aggressiveness and metastasis in breast cancer xenografts (Wallach-Dayan et al., 2008). It was identified that the cDNA vaccine induced the production of antibodies recognizing CD44 variant isoforms (Wallach-Dayan et al., 2008). In addition, several studies revealed the beneficial effect of CD44-targeting with micro-RNA (miRNA). MiR-199a overexpression in CD44+ cancer-initiating cells (CICs) in ovarian cancer xenografts significantly decreased tumor volume (Cheng et al., 2012). Additionally, it was identified that a more significant percentage of apoptotic cells is formed with miR-199a overexpression. Similar effects were observed with the overexpression of miR-24a and miR-34a in gastric and esophageal cancer xenografts (Jang et al., 2016; Zuo et al., 2018). Overall, the data surrounding gene therapy approaches seems promising regarding cancer patient treatment, but they should be cautiously studied since they can carry the risk of off-target effects.
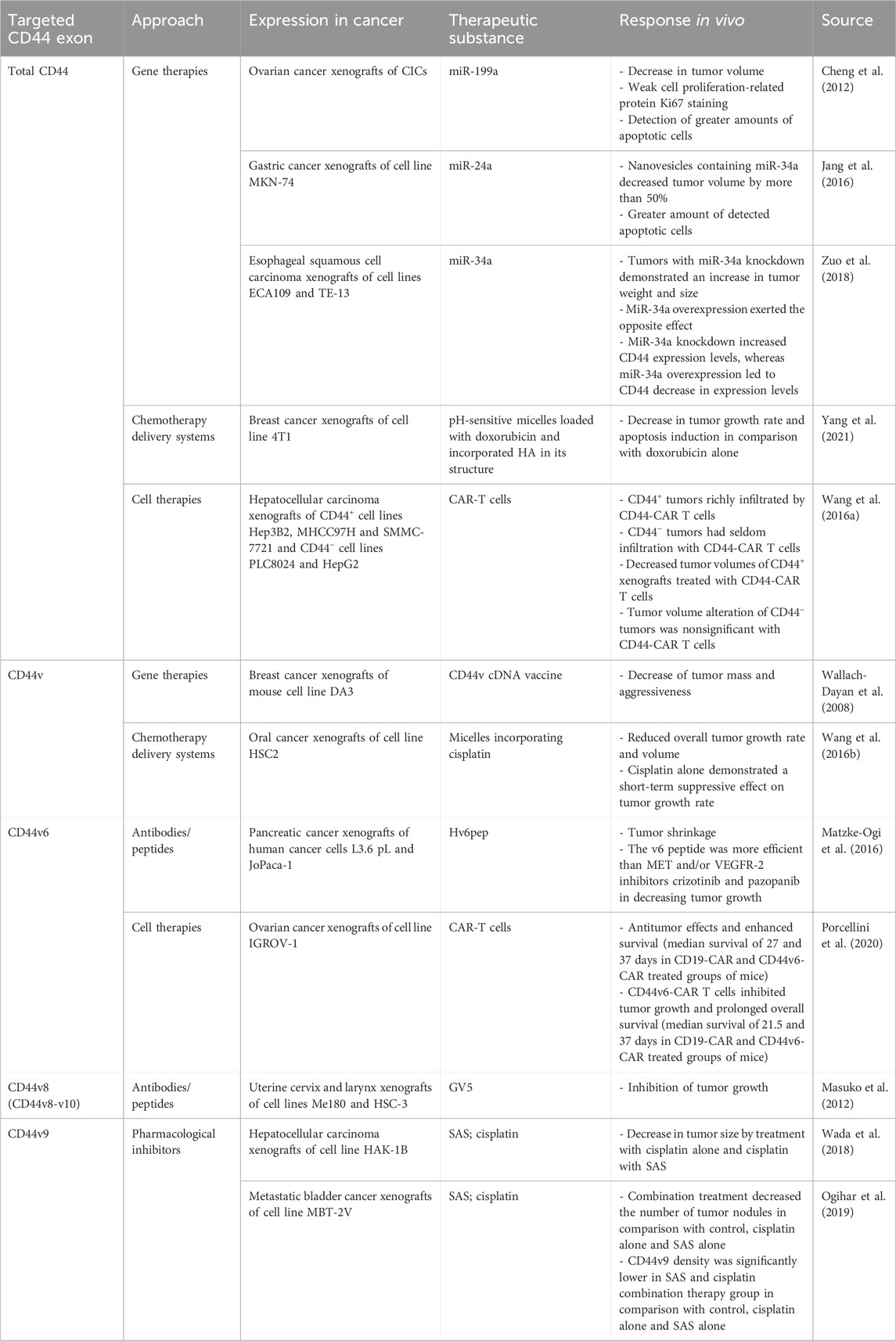
Table 4. Summary of in vivo studies using CD44-targeted therapy.
Moreover, CD44 variant isoforms can be helpful for tumor cell targeting (cell therapies) and chemotherapy delivery due to their overexpression in tumor tissue. Anti-CD44 variant isoforms micelles, incorporating cisplatin, have shown success in decreasing the tumor volume of oral cancer xenografts by more than 50% (Wang et al. (2016a)). In addition, CD44-targeted and pH-sensitive doxorubicin-loaded micelles have exerted selective cytotoxic effects in breast cancer xenografts, resulting in apoptosis induction (Yang et al., 2021). A recently developed method for tumor cell targeting is therapy with chimeric antigen receptor (CAR) T cells, successfully applied for CD44+ tumor cell elimination. For instance, CD44+ hepatocellular carcinoma xenografts treated with CD44-CAR T cells demonstrated a significant decrease in tumor volume in comparison with mock T and normal T cells; CD44 tumor volumes were unchanged in comparison with CD44-CAR T, mock T and normal T cells (Wang et al., 2020).
Even though preclinical research data suggest that total CD44 play a significant role in the chemoresistance of cancer patients, there is a limited amount of conducted clinical studies regarding total CD44 targeted therapies (Table 5). They include therapeutic approaches such as using antibodies/peptides or pharmacological inhibitors.
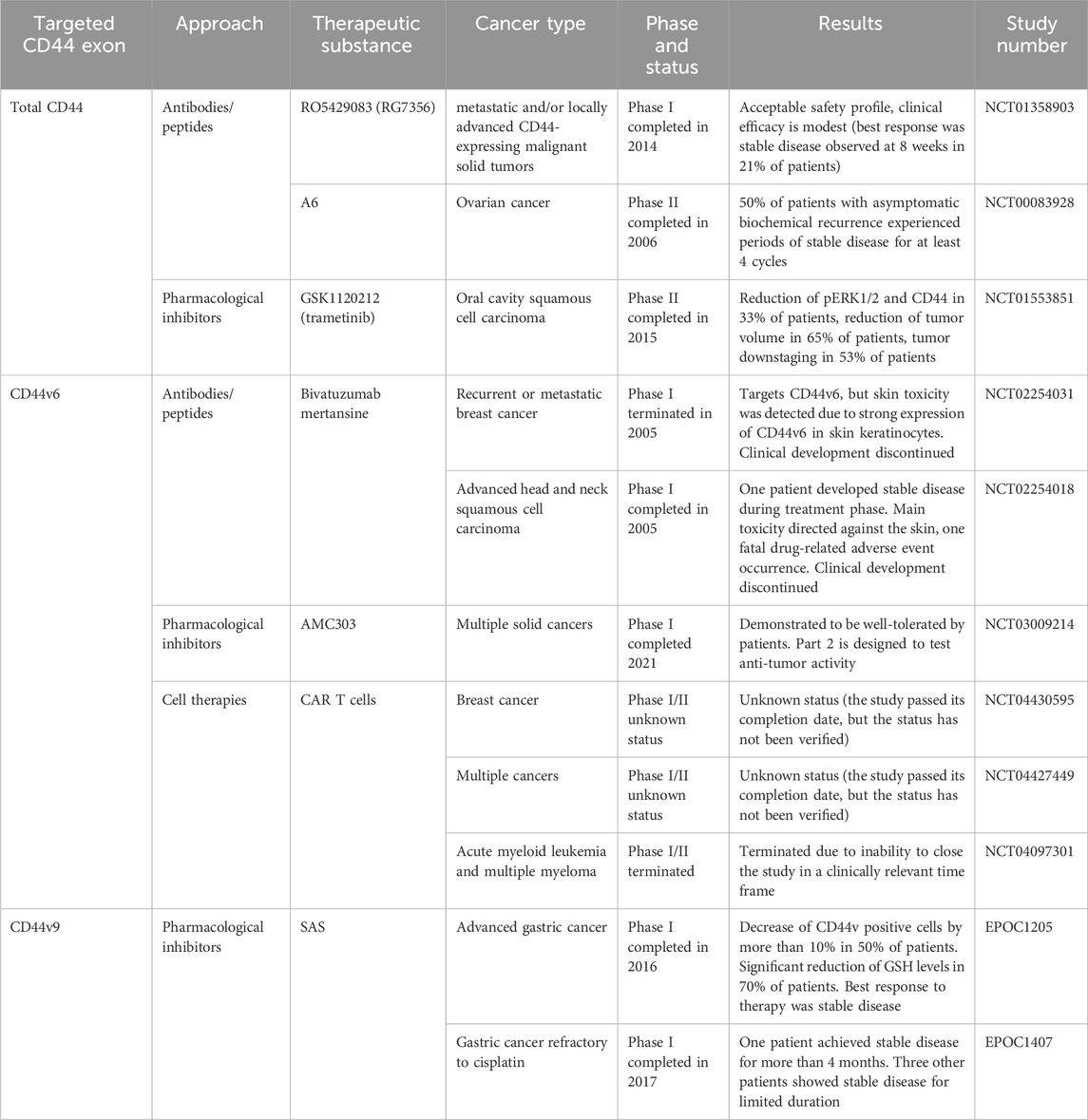
Table 5. Clinical trials aimed at targeting CD44 for overcoming chemoresistance. Retrieved from clinicaltrials.org and umin.ac.jp/ctr.
Anti-total-CD44 targeting approaches with modest/insufficient effect have been noted (NCT01358903): an anti-CD44 antibody RO5429083 (alternatively named RG7356) was tested in metastatic and/or locally advanced CD44-expressing malignant solid tumors (Menke-van der Houven van Oordt et al., 2016). The developed antibodies demonstrated an acceptable safety profile, however, clinical efficacy was identified to be modest–the best response to chemotherapy was stabilization of disease progression identified in 21% of patients. Some promising clinical trials have also been conducted (NCT00083928 and NCT01553851). A6 peptide, a short amino acid sequence targeting the HA-binding domain of CD44, performed well in increasing the time to progression of patients with ovarian cancer: half of the patients with asymptomatic biochemical recurrence experienced periods of stable disease for at least 4 cycles of administered A6 peptide therapy (Ghamande et al., 2008). Moreover, trametinib (conversely named GSK1120212) was shown to decrease Ras/MEK/ERK pathway activation and CD44 expression, resulting in a beneficial clinical response in patients (the effect was seen in 11 out of 17 patients), a decrease in tumor volume and tumor downstaging (Figure 4; Uppaluri et al., 2017).
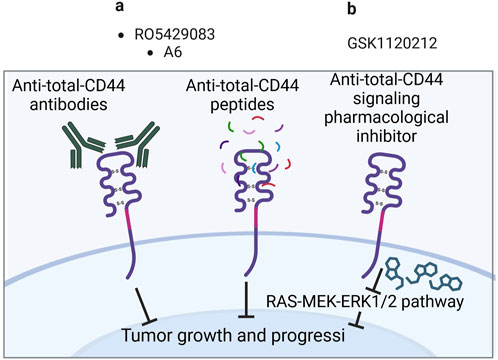
Figure 4. Clinical trials aimed at targeting total CD44 (Created with bioRender.com). (A) Antibodies/peptides. (B) Pharmacological inhibitors.
6 CD44 variant exon 3 and chemoresistance6.1 Effect of CD44 variant exon v3 expression on chemotherapy treatment outcome/tumor response to chemotherapy in patients/animal studiesCD44v3 seems to be the least studied variant exon out of the group of clinically relevant exons (Table 6). Concerning the correlation of expression levels with clinical outcomes of patients, it was demonstrated that high protein expression levels of CD44v3 exert chemoradiotherapy resistance in a study with nasopharyngeal patients (Sagawa et al., 2016). There seem to be no studies examining the association between CD44v3-containing isoforms overexpression/knockdown and chemoresistance in vivo. Nevertheless, the biological effect of CD44v3 has been studied in vitro.
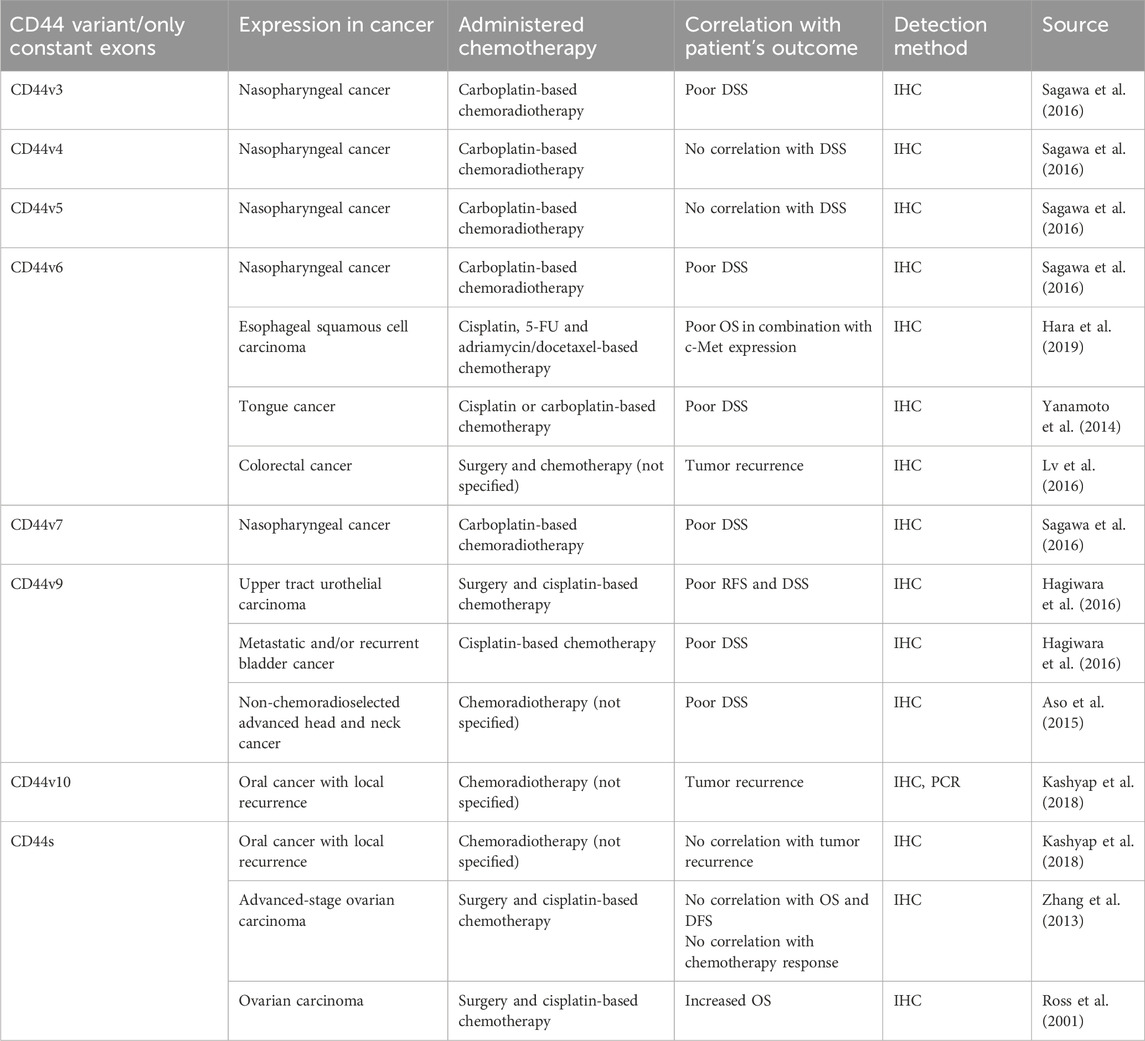
Table 6. The association of CD44 variant/only constant exon expression levels with clinical outcomes of cancer patients.
6.2 CD44 variant exon v3 biological functions in relation to cell death pathwaysThe biological effects exerted by variant exons of CD44 with significant clinical association concerning cell death pathways are summarized in Table 3. They will be covered in detail in the upcoming sections of our review.
The study of a particular exon’s functional role is quite tricky. Such methods as overexpression/knockdown of particular CD44 isoforms, which typically contain more than one variant exon, are widely applied. Indeed, we are not the first to review the involvement of CD44 variant exons in chemoresistance induction through apoptosis regulation (Wang et al., 2018). However, Wang et al. primarily focus on variant exon v6. In our review, we outline what is known to date about all clinically relevant variant exons regarding cell death mechanisms, including apoptosis, ferroptosis and autophagy.
Firstly, CD44 has been shown to bind some metalloproteinases (MMPs), a family of endopeptidases that hydrolyze components of the extracellular matrix (Hassn Mesrati et al., 2021). Specifically, CD44v3 containing isoforms were identified to recruit the active form of MMP-7 and the precursor of HB-endothelial growth factor (HB-EGF) at its extracellular domain. This is possible due to HB-EGF binding of HS-rich sites in variant exon v3 of CD44 (Yu et al., 2002). Such an interaction allows the presentation of mature HB-EGF to membrane receptors, such as ERBB4, and thus the promotion of cell survival (Figure 5A; Yu et al., 2002). The mechanism through which CD44 forms complexes at the cellular membrane with additional receptors is quite often utilized by cancer cells and seems to be successful in the promotion of cell death resistance.
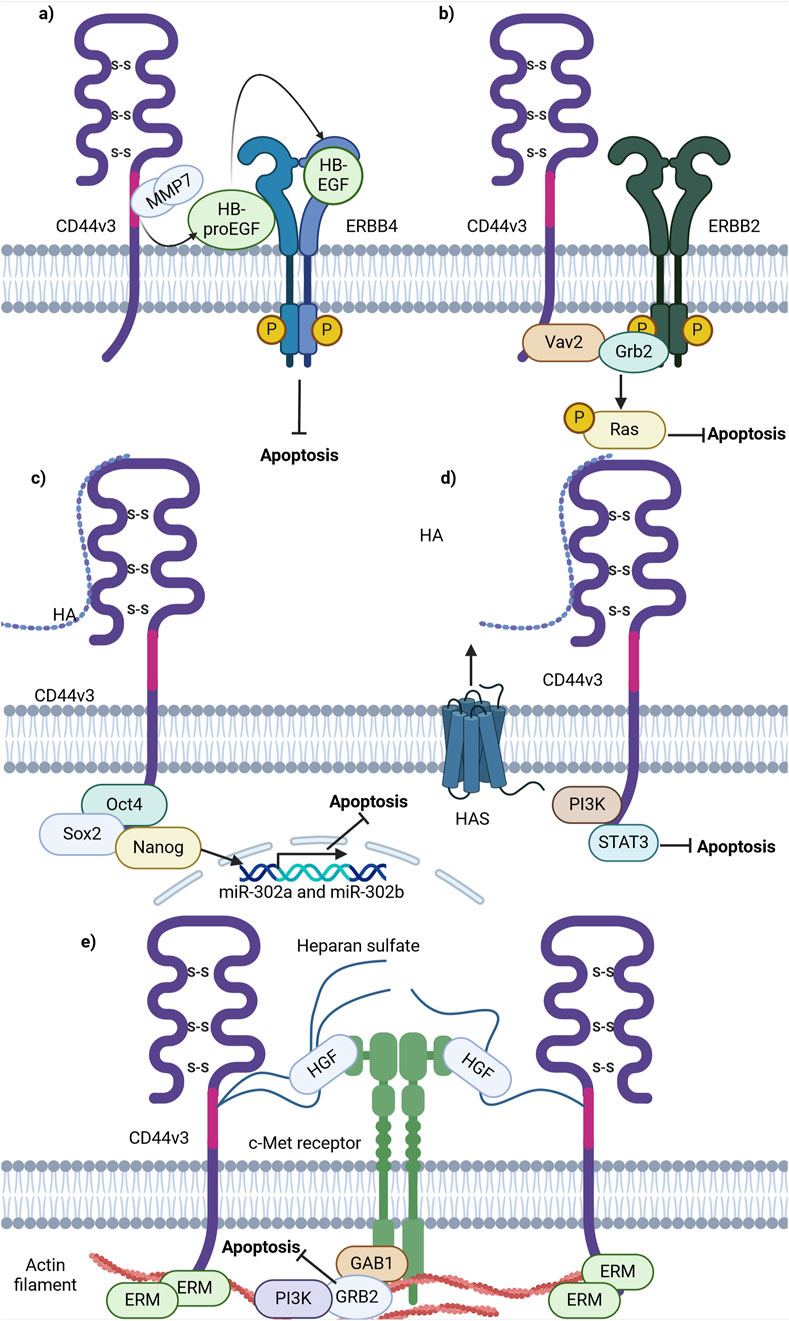
Figure 5. Schematic representation of CD44 variant exon v3 interaction with cellular receptors/intracellular proteins involved in cell death signaling. (A) CD44v3 containing isoforms recruit MMP7 and inactive HB-EGF, leading to consequent cleavage, activation and presentation of HB-EGF to ERBB4, thereby promoting anti-apoptotic signaling. (B) CD44v3 containing isoforms form a complex with ERBB2, Vav2 and Grb2 and promote anti-apoptotic Ras-MAPK signaling. (C) The ICD of CD44v3 containing isoforms promotes the formation of Oct4, Sox2 and Nanog complex and its consequent shift to the nucleus. The intranuclear Oct4/Sox2/Nanog complex promotes the expression of miR-302a and miR-302b, promoting anti-apoptotic signaling. (D) CD44v3 promotes antiapoptotic signaling through PI3K/STAT3 pathway. (E) CD44v6 containing isoforms activate c-Met anti-apoptotic signaling: upon binding of HGF, CD44v6 form dimeric complexes (not shown in figure) that are able to laterally diffuse through the plasma membrane for rapid interaction with c-Met with the formation of an active trimeric complex (Created with BioRender.com).
Moreover, CD44v3-containing isoforms were shown to promote ovarian tumor growth through ERBB2 signaling. It was identified that the ICD of CD44v3-containing isoforms interacts with adaptor proteins Vav2 and growth factor receptor bound protein 2 (Grb2) (Bourguignon et al., 2001), thus promoting coupling of ERBB2 and CD44v3-containing isoforms to Ras and the promotion of its signaling (Bourguignon et al., 2001; Figure 5B). This interaction is similar to that of total CD44 and ERBB2, but the mechanisms of apoptosis promotion vary. This points to a nonuniform response of tumor cells from different cancers upon interaction of CD44 with the same receptor.
Additionally, CD44v3 was shown to promote apoptosis resistance through epigenetic regulatory mechanisms. For instance, in CSCs with a CD44v3high/ALDH1high phenotype, master transcriptional factors Oct4, Sox2 and Nanog were detected to be overexpressed at the mRNA and protein levels (Bourguignon et al., 2012). Interestingly, upon activation of CD44v3-containing isoforms by HA in such cells, CD44v3-associated Oct4, Sox2 and Nanog physical complex formation and dislocation of Oct4/Sox2/Nanog to the nucleus was observed (Bourguignon et al., 2012). Upon nuclear localization, Oct4/Sox2/Nanog was identified to bind the miR-302 cluster promoter region, enhancing the production of miR-302a and miR-302b expression. SiRNA-mediated knockdown of these factors blocked HA-mediated Oct4/Sox2/Nanog binding to the miR-302 cluster promoter and decreased HA-induced antiapoptotic IAP protein expression, promoting chemosensitivity to cisplatin (Bourguignon et al., 2012). Thus, these finding indicate the ability of CD44v3 to utilize regulatory mechanisms not only at the protein level, but also epigenetically.
Further investigations into the role of CD44v3 in apoptosis regulation unveiled that the knockdown of CD44v3-containing isoforms mediates the reduction of PI3K, pAKT, pERK, pSTAT3 and Bcl2 protein levels, triggering apoptotic signaling and G0/G1 phase arrest in bladder cancer cells (Anand et al., 2019). Combinational treatment of cisplatin and doxorubicin with 4-methylumbelliferone (4-MU), an HA synthesis inhibitor, significantly decreased cell viability compared to cisplatin or doxorubicin alone, suggesting that the HA/CD44v3 axis is of importance in apoptotic signaling regulation (Figure 5D; Anand et al., 2019).
As mentioned previously, CD44v3 encodes a heparan sulphate (HS) side-chain attachment motif, allowing HS-bound CD44v3 to bind growth factors including hepatocyte growth factor (HGF) and to present them to a number of cell membrane receptors including the mesenchymal epithelial transition factor receptor (c-Met) (van der Voort et al., 1999). A significant pathway of c-Met signaling is the PI3K/Akt signaling axis primarily responsible for the cell survival response: the p85 subunit of PI3K can bind either directly to c-Met or indirectly through GAB1, which in turn promotes anti-apoptotic signaling through AKT (Xiao et al., 2001). Thus, these findings underline the importance of HS motifs and their utilization by CD44v3-containing isoforms to promote antiapoptotic signaling.
Overall, CD44v3-containing isoforms contribute to apoptosis resistance through several mechanisms, including the c-Met and ERBB2 receptors, epigenetic regulation employing miR-302 cluster upregulation and MMPs. Further research on these pathways will facilitate the development of targeted therapies to overcome chemoresistance and improve cancer treatment outcomes.
6.3 CD44 variant exon v3 therapeutic strategies and clinical implicationsDespite the relatively large number of molecular mechanisms of resistance in which CD44v3 partakes, there seem to be practically no antibodies or clinical trials aimed at targeting CD44v3. A novel monoclonal antibody, C44Mab-6, was recently established (Suzuki et al., 2023). One advantage of such antibodies is the area of recognition of CD44v3-containing isoforms. For instance, compared with previously established anti-CD44v3 antibodies (clone 3G5), C44Mab-6 recognizes a peptide sequence excluding an HS-modified sequence in the CD44 variant-3-encoded region, indicating that C44Mab-6 antibodies recognition of CD44v3 is not influenced by HS modifications (Suzuki et al., 2023). This may be useful for CD44v3 targeting, increasing the selectivity of such antibodies. The authors mention that further testing of C44Mab-6 in vivo is reckoned.
Apart from the development of the antibodies mentioned above, it does not appear that other antibodies have been synthesized and tested in clinical settings. Thus, further research is necessary to determine the relevance of CD44v3 inhibition, considering the small number of studies regarding the analysis of its clinical relevance.
7 CD44 variant exon v6 and chemoresistance7.1 Effect of CD44 variant exon v6 expression on chemotherapy treatment outcome and survival of patients and tumor response to chemotherapy in animal studiesOne of the most widely and thoroughly studied variant exons concerning the clinical outcome of patients and biological effect is CD44v6 (Table 6). Several studies unveiled the prognostic significance of CD44v6 in nasopharyngeal, esophageal (in combination with c-Met expression) and tongue cancer (Hara et al., 2019; Sagawa et al., 2016; Yanamoto et al., 2014). High protein expression levels of CD44v6 significantly correlated with poor disease-specific survival (DSS) and OS of patients treated with cisplatin/carboplatin-based chemotherapy, as well as with tumor recurrence after chemotherapy treatment in colorectal cancer patients (Lv et al., 2016).
In addition, the association between CD44v6 overexpression/knockdown and chemoresistance in vivo has been studied (Table 2). Research indicates that CD44v6kd in prostate and colon cancer xenografts demonstrated a significant decrease in tumor growth and practically complete impalpability by the end of docetaxel administration and in folinic acid, 5-FU and oxaliplatin (FOLFOX)-resistant cells (Ni et al., 2020; Ghatak et al., 2021).
Overall, the implemented literature analysis, although not comprehensive, pointed to a relationship between variant exon CD44v6 high expression levels and worse chemotherapy treatment outcomes in cancer patients. Cancer xenografts confirmed the negative relationship of CD44v6 expression level with worse chemotherapy efficacy in vivo. The results demonstrate that CD44v6 may be involved in chemotherapy resistance, and further research into its molecular mechanisms is needed.
7.2 CD44 variant exon v6 biological functions in relation to cell death pathwaysThe ongoing investigation into the role of CD44 variant exon v6 in cellular death pathways across various cancer types may not yet provide a comprehensive understanding for all cancers. To date, CD44v6 is the most studied variant exon out of the other clinically variant exons. It has been shown to influence apoptosis and autophagy.
One of the pioneering studies on CD44v6 involvement in apoptosis revealed that CD44 exon v6 containing isoforms exhibit antiapoptotic properties through colocalizing, interacting with and blocking FAS trimerization (Figure 6A; Mielgo et al., 2006). Notably, cells, constitutively expressing FASR and being sensitive to apoptosis with the addition of FASL or FAS-crosslinking antibodies, become resistant to apoptosis with the transfection of CD44 variant isoforms. It was demonstrated that blocking the CD44v6 exon with anti-CD44v6 antibodies (clone VFF18) in cancer cells results in their sensitization to apoptosis, thereby underscoring CD44v6’s role in the promotion of antiapoptotic signaling. The authors further propose that CD44 variant isoforms may interact with the pre-ligand assembly domain of FASR and prevent its trimerization, which is necessary for FASL binding (Mielgo et al., 2006). Thus, CD44v6-containing isoforms employ regulatory mechanisms acting directly on the extrinsic apoptotic signaling pathway.
Comments (0)