Tralokinumab, a humanized monoclonal antibody targeting interleukin-13 (IL-13), represents a significant advancement in treating moderate-to-severe atopic dermatitis (Müller et al., 2024). With the increasing global prevalence of atopic dermatitis, this condition significantly impairs patients’ quality of life and places substantial burdens on healthcare systems (Langan et al., 2020). The introduction of tralokinumab permitted a targeted therapeutic approach that aligns with the pathophysiological mechanisms of atopic dermatitis, marking a new era in managing this chronic skin disease (Popovic et al., 2017; Wollenberg et al., 2019; Zhang et al., 2022).
Tralokinumab exerts its therapeutic effects by neutralizing the activity of IL-13, a crucial cytokine in the inflammatory cascade of atopic dermatitis (Popovic et al., 2017; Zhang et al., 2022). Its efficacy has been validated in numerous Phase II and III clinical trials, demonstrating significant improvements in the eczema area and severity index, investigator’s global assessment score, dermatology life quality index, and symptoms such as itching and sleep disruption (Wollenberg et al., 2019; Wollenberg et al., 2021; Gutermuth et al., 2022). Although the short-term safety profile of tralokinumab has been established through multiple studies (Silverberg et al., 2021; Gutermuth et al., 2022), a comprehensive evaluation of its long-term safety in clinical practice is lacking.
The FDA Adverse Event Reporting System (FAERS) compiles drug adverse event reports voluntarily submitted by consumers, physicians, and pharmacists. It serves as an essential resource for assessing the real-world safety of medications (Shu et al., 2022a; Shu et al., 2022b). Recently, FAERS was used to identify adverse events associated with various drugs, such as antiviral drug-associated DRESS syndrome (Sharma and Kumar, 2022; Sharma et al., 2023) and the effects of proton pump inhibitors on the renal system (Jain et al., 2023).
This study assessed the safety profile of tralokinumab in real-world settings by analyzing data from the FAERS database through disproportionality analysis. The findings could provide clinicians with guidance on the safe administration of this medication.
2 Materials and methods2.1 Data sources, management, and study designThis study used data from the publicly accessible FAERS database, a spontaneous reporting system in which reports are primarily submitted by consumers, physicians, and pharmacists. The analysis included all adverse event reports in which tralokinumab was identified as the primary suspected drug, and the search period spanned from the fourth quarter of 2021 to the fourth quarter of 2023. The data management process involved removing duplicate reports and standardizing adverse event terminology. Duplicates were processed according to FDA-recommended practices. Specifically, for reports with identical case identifiers (CASEIDs), reports with the highest FDA receipt date (FDA_DT) were retained. When the CASEID and FDA_DT values matched, the report with the highest PRIMARYID (the unique identifier assigned to each report) was retained. Adverse event terms were standardized using the MedDRA dictionary, version 26.1, thereby enhancing the reliability of subsequent statistical analyses. Figure 1 presents a detailed flowchart of the study design.
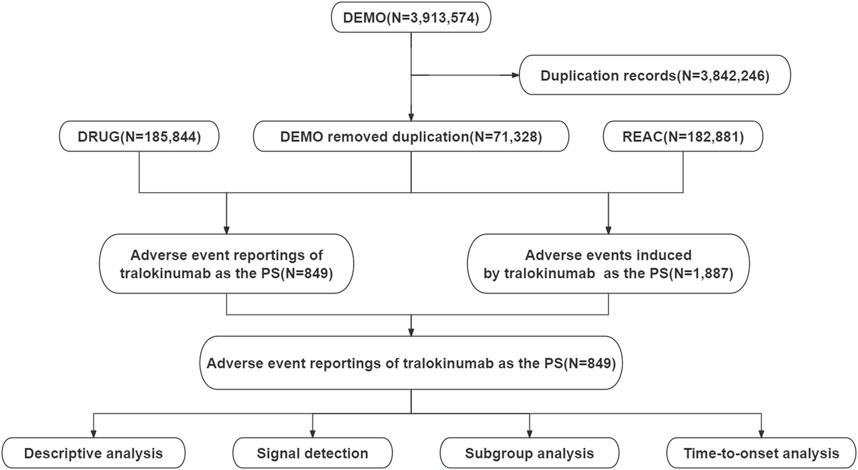
Figure 1. Flowchart demonstrating the adverse event analysis process for tralokinumab using the FDA Adverse Event Reporting System database.
2.2 Statistical analysisDescriptive analysis was employed to characterize the features of adverse event reports associated with tralokinumab. Four disproportionality analysis methods were used to detect signals of potential adverse reactions attributable to tralokinumab: reporting odds ratio (ROR) (Rothman et al., 2004), proportional reporting ratio (PRR) (Evans et al., 2001), multi-item gamma Poisson shrinker (MGPS) (Rivkees and Szarfman, 2010), and Bayesian confidence propagation neural network (BCPNN) (Ang et al., 2016). An adverse event meeting the positivity threshold for at least one method was considered a potential adverse reaction. Detailed two-by-two contingency tables are provided in Supplementary Table S1. The formulas and thresholds for these disproportionality analyses are outlined in Supplementary Table S2. The interval between the occurrence of adverse events (as reported in the DEMO file) and the initiation of tralokinumab treatment (as recorded in the THER file) defined the onset time of tralokinumab-related adverse events. The Weibull distribution was applied to model changes in the incidence of adverse events over time. All analyses were performed using R software version 4.2.2.
3 Results3.1 Descriptive analysisThis investigation encompassed 849 reports of adverse events (1887 total adverse events) in which tralokinumab was the suspected primary agent. Of these, 60.5% involved female patients, whereas 38.5% involved male patients. The predominant age group was 18–65 years, accounting for 40.3% of the cases. Most reports (65%) were submitted by healthcare professionals, with 92.3% originating from the United States, followed by 1.9% from Germany and 1.6% from Canada. Further descriptive outcomes are presented in Table 1.
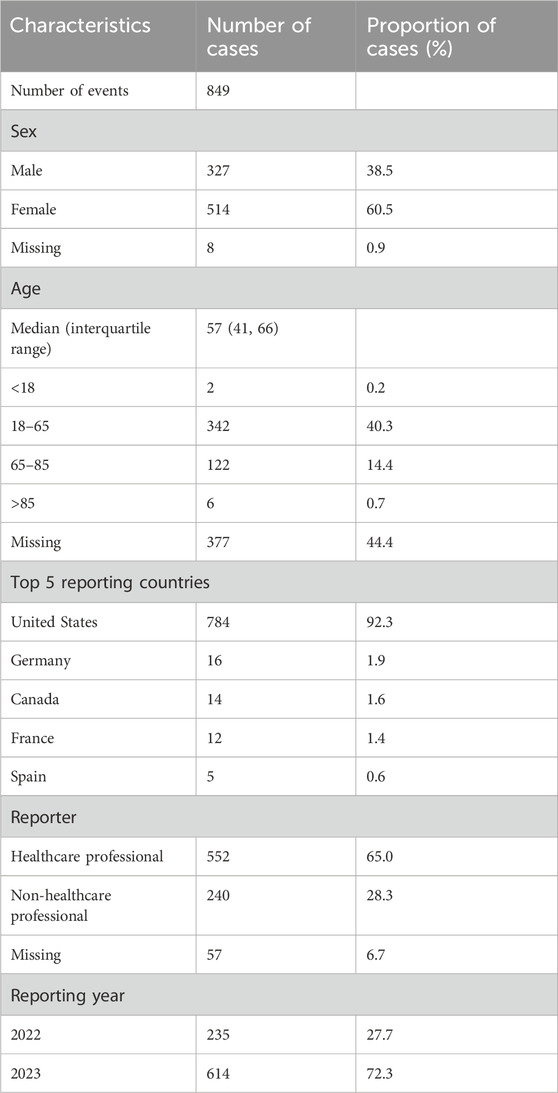
Table 1. Clinical characteristics of adverse event reports related to tralokinumab from the FDA Adverse Event Reporting System database (Q4 2021–Q4 2023).
3.2 Distribution of adverse events at the system organ class (SOC) levelAdverse events associated with tralokinumab encompassed 25 of the 27 SOCs. As presented in Table 2, significant findings were observed in several categories, including general disorders and administration site conditions, gastrointestinal disorders, nervous system disorders, infections and infestations, eye disorders, vascular disorders, surgical and medical procedures, and congenital, familial, and genetic disorders. The distribution of adverse events at the SOC level is depicted in Figure 2.
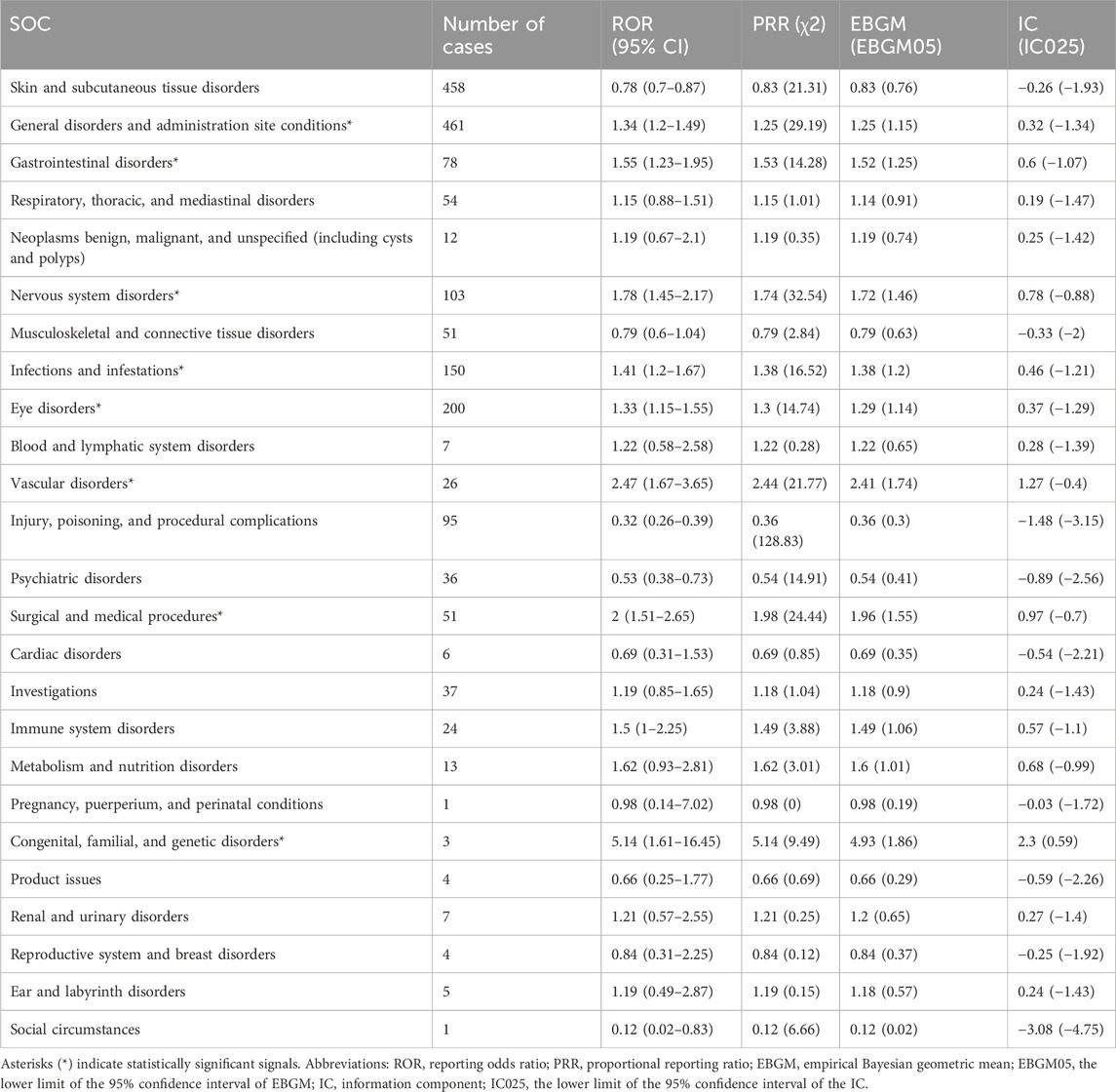
Table 2. Signal strength of tralokinumab-related adverse events across system organ classes (SOCs) in the FDA Adverse Event Reporting System database.
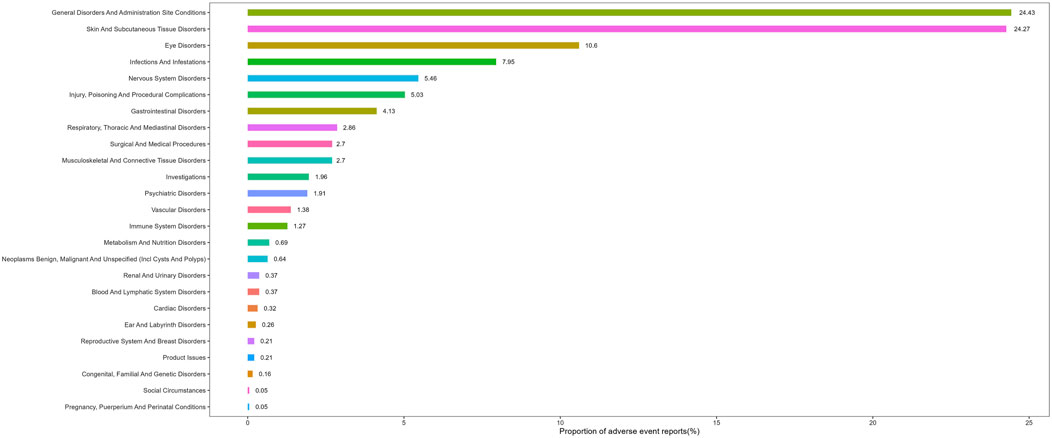
Figure 2. Proportion of adverse events by system organ class for tralokinumab.
3.3 Distribution of adverse events at the preferred term (PT) levelAdverse events associated with tralokinumab were ranked by frequency and evaluated for positive signals. Among the 50 most common adverse events, known reactions including injection site erythema, injection site pruritus, conjunctivitis, hypersensitivity, and nasopharyngitis were confirmed. Furthermore, potential adverse reactions not listed on the label, such as headache, fatigue, dizziness, urticaria, nausea, vomiting, hair loss, syncope, and urinary tract infections, were additionally identified. Detailed information on these findings is presented in Table 3. All adverse events that met the criteria for a positive signal are documented in Supplementary Table S3.
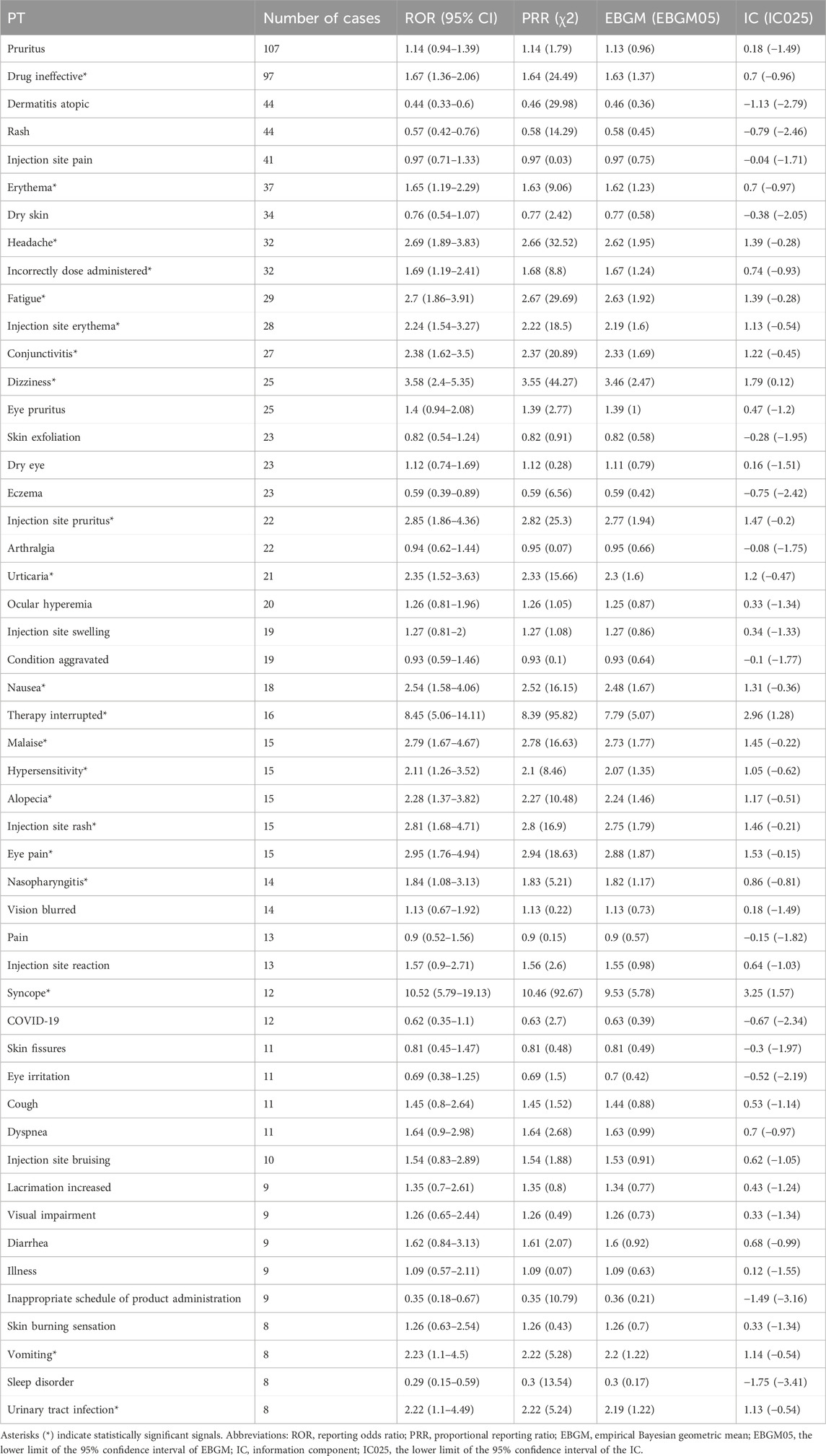
Table 3. Top 50 most frequent adverse events for tralokinumab at the preferred term (PT) level.
3.4 Subgroup analysisSubgroup analysis of adverse events associated with tralokinumab revealed that among the 50 most common adverse events meeting the positive signal criteria, events occurring exclusively in males included diarrhea and vomiting, whereas acne and muscle spasms were specific to females. Specific details are presented in Supplementary Tables S4, S5. Only two adverse event reports involved patients younger than 18 years, and they did not feature events beyond those specified on the drug label. In patients aged 18–65 years, the 50 most frequent adverse events displaying positive signals included headache, dizziness, syncope, fatigue, nausea, vomiting, diarrhea, hair loss, and urticaria. For patients older than 65 years, common adverse events included fatigue, urinary tract infection, and facial swelling (Supplementary Tables S6–S8).
3.5 Sensitivity analysisTralokinumab is commonly used in combination with medications such as prednisone, budesonide, clobetasol, tacrolimus, and pimecrolimus. After excluding reports involving the concurrent use of other drugs, we identified 806 reports involving 1733 adverse events. Persistent potential adverse reactions included headache, fatigue, dizziness, hives, nausea, hair loss, fainting, and urinary tract infections (Supplementary Table S9).
3.6 Time to onset and weibull distribution analysis of adverse eventsRegarding the time to onset, adverse events associated with tralokinumab primarily occurred within the first month of treatment. The specific temporal distribution of these events is depicted in Figure 3. Additionally, the cumulative incidence curve of adverse events is illustrated in Figure 4. Analysis using the Weibull distribution indicated an early failure mode. The detailed parameters of this analysis are provided in Table 4.
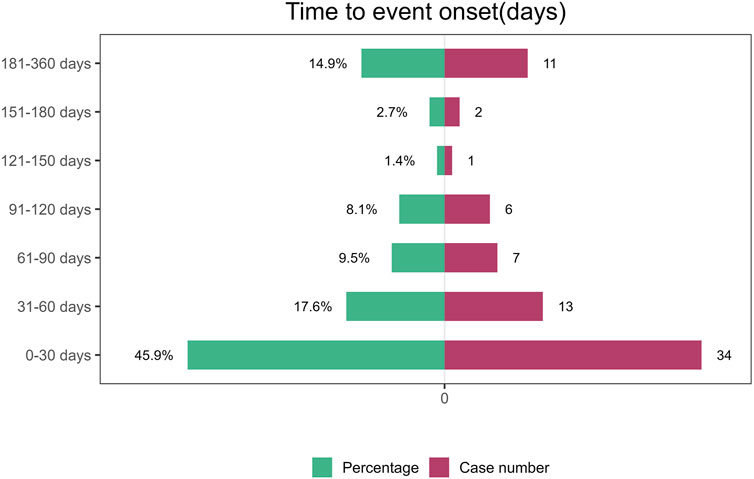
Figure 3. Time to onset of adverse events induced by tralokinumab.
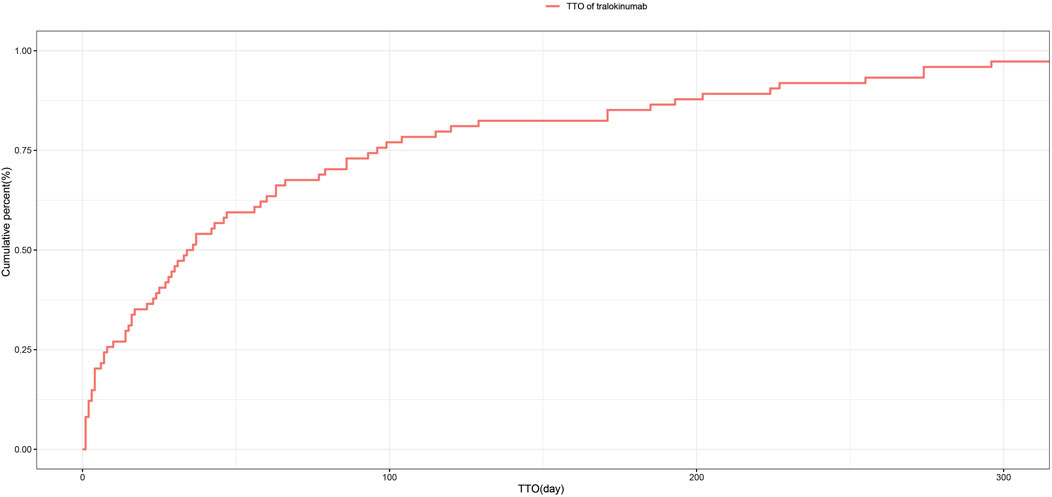
Figure 4. Cumulative incidence of adverse events related to tralokinumab over time.

Table 4. Time to onset of tralokinumab-associated adverse events and Weibull distribution analysis.
4 DiscussionThis study comprehensively assessed adverse events associated with tralokinumab following its market launch in 2021. By analyzing data from FAERS, this research confirmed previously identified adverse reactions included on the tralokinumab drug label, including injection site reactions, conjunctivitis, hypersensitivity, upper respiratory infections, and nasopharyngitis. Additionally, adverse events not cited on the label, including neurological symptoms (e.g., dizziness, headache, syncope), gastrointestinal disturbances (e.g., nausea, vomiting), and dermatological reactions (e.g., urticaria, hair loss), were also uncovered. These findings emphasize the need for drug monitoring, particularly within the first month of treatment initiation, to effectively manage and mitigate potential adverse effects.
Multiple clinical trials describe conjunctivitis as a common adverse reaction of tralokinumab. For instance, a Phase II trial of tralokinumab in the treatment of atopic dermatitis reported conjunctivitis rates of 5.9% at a dosage of 150 mg and 2% at a dosage of 45 mg (Wollenberg et al., 2019). Further, an analysis of five clinical trials involving 2,285 adults with moderate-to-severe atopic dermatitis detected a higher incidence of conjunctivitis in the tralokinumab group (7.5%) than in the placebo group (3.2%) (Wollenberg et al., 2022). Our findings are consistent with previous research. It is crucial to monitor and manage this adverse reaction vigilantly when administering tralokinumab for atopic dermatitis.
Another adverse reaction warranting attention is upper respiratory infection. Consistent with our results, the incidence of upper respiratory infections was higher for tralokinumab than for placebo in both 16- and 52-week clinical trials (Silverberg et al., 2021; Wollenberg et al., 2021). This adverse reaction has also been observed in patients who received tralokinumab for asthma. Although tralokinumab effectively reduces airway inflammation and mucus secretion in asthma, potentially alleviating symptoms (Brightling et al., 2015), it can also induce treatment-emergent adverse events such as upper respiratory infections (Busse et al., 2019). Upper respiratory infections can decrease medication adherence among patients. Therefore, when using tralokinumab to treat atopic dermatitis, clinicians should be vigilant regarding the possibility of upper respiratory infections.
Additionally, our study revealed several adverse events not currently listed on the drug label. Notably, tralokinumab can induce neurological reactions, including headache and dizziness. The precise mechanism by which tralokinumab triggers headache remains to be elucidated. Although not documented as an adverse reaction on the drug label, headache was frequently reported in multiple clinical trials of tralokinumab for treating atopic dermatitis (Wollenberg et al., 2021). Moreover, dizziness could be a manifestation of underlying vascular conditions. For instance, in a case report of dupilumab, a monoclonal antibody targeting IL-4Rα used to treat atopic dermatitis, the patient developed ischemic stroke characterized by dizziness and nausea. This condition was possibly exacerbated by the inhibition of IL-4 and IL-13 by dupilumab, resulting in the activation of the pro-inflammatory cytokines IL-1β and TNF-α in endothelial cells, thereby activating coagulation pathways and promoting thrombus formation (Iwase et al., 2020). Similarly, tralokinumab’s specific inhibition of IL-13 might precipitate adverse events akin to those observed with dupilumab, though the exact pathways for tralokinumab-induced headache and dizziness warrant further investigation. These neurological adverse events can profoundly affect patients’ quality of life, especially among individuals susceptible to atopic dermatitis (Lugović-Mihić et al., 2023). Therefore, these unlisted adverse events should be closely monitored and seriously considered by clinicians.
Nausea and vomiting are potential adverse reactions not mentioned on the drug label that could limit the clinical use of tralokinumab. These gastrointestinal symptoms can cause substantial physiological and psychological distress, potentially leading to treatment discontinuation. It is therefore imperative to meticulously monitor patients’ responses following tralokinumab administration. Timely intervention with antiemetic medications such as ondansetron can effectively control these symptoms and enhance patient adherence to the treatment regimen.
Additionally, this study identified hair loss as a potential adverse reaction of tralokinumab. Notably, atopic dermatitis affects both children and a significant proportion of adults. In high-income countries, the incidence of atopic dermatitis among adults is approximately 10%, and its prevalence is increasing globally (Langan et al., 2020). Research indicates that hair loss in adults can have extensive psychological and emotional repercussions extending beyond physical symptoms (Gorbatenko-Roth et al., 2023; Muntyanu et al., 2023; Clemmesen et al., 2024). Hair loss can lead to anxiety, depression, social withdrawal, and reduced self-esteem, which can severely affect patients’ overall quality of life and work productivity (Gandhi et al., 2023). Given that hair loss represents a potential adverse reaction not listed on the drug label, it is crucial for patients receiving tralokinumab treatment for atopic dermatitis to be preemptively informed about the risk of hair loss. Proactive management of this and other adverse reactions is essential to enhance the overall quality of life of patients.
The subgroup analysis emphasized that gastrointestinal symptoms, such as diarrhea and vomiting, should receive additional attention in males, whereas vigilance regarding the risks of acne and muscle spasms is needed in females. Notably, acne can cause significant psychological stress, especially in females, potentially leading to anxiety, depression, and even suicidal thoughts (Altunay et al., 2020; Hughes and Bewley, 2023). In the pediatric population, no adverse reactions were observed beyond those described in the drug label. In elderly patients older than 65, vigilance is warranted concerning urinary tract infections, which can adversely affect their quality of life. Through sensitivity analysis, we identified persistent potential adverse reactions associated with tralokinumab monotherapy, including headache and fatigue. Such non-lethal but impactful adverse events can influence treatment adherence, adversely affecting therapeutic efficacy. Given these findings, it is crucial to pay careful attention to these specific adverse events to optimize treatment outcomes and enhance medication effectiveness.
This study additionally conducted a temporal analysis of adverse events and employed the Weibull distribution to predict the timing of these events, facilitating the establishment of effective timelines for monitoring drug-related adverse reactions. The results underscore the critical importance of vigilant monitoring, particularly during the first month of tralokinumab treatment. This early monitoring period is crucial for detecting and managing potential adverse reactions, thereby optimizing patient safety and treatment outcomes.
This study had several limitations. First, the FAERS database, as a spontaneous reporting system utilized by consumers, physicians, and pharmacists, can inherently contain missing or inaccurate data. For example, data related to patient exposure to medicines are not available. Second, the volume of data analyzed in this study was limited, and larger datasets are necessary to validate our findings. However, our study both focused on the medication itself and specifically addressed its indications for use, thereby enhancing the specificity of the results. Third, the majority of the data were sourced from the United States, which might have introduced reporting bias. Future research should aim to incorporate data from multiple countries to enhance generalizability. Lastly, although disproportionality analysis effectively identified positive signals of adverse events, it did not establish a causal relationship between tralokinumab and these events. Long-term prospective studies are necessary to confirm the potential adverse reactions identified in this research.
5 ConclusionIn this study, we conducted a comprehensive analysis of adverse events associated with tralokinumab using the FAERS database, focusing on reports published since 2021. The analysis both confirmed known adverse reactions and identified potential adverse reactions not mentioned on the label, such as dizziness, headache, nausea, vomiting, hair loss, and acne. These findings offer essential safety information for clinicians prescribing tralokinumab and underscore the necessity to closely monitor patients for potential adverse reactions during the treatment of atopic dermatitis.
Data availability statementThe datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Ethics statementEthical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributionsKZ: Conceptualization, Data curation, Methodology, Writing–original draft. YZ: Data curation, Writing–original draft. SX: Supervision, Writing–review and editing. CT: Writing–review and editing, Funding acquisition.
FundingThe author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant No. 82003356), the Natural Science Foundation of Shaanxi Province (Grant No. 2019JQ-511), and the Basic-Clinical Fusion Innovation Project of Xi’an Jiaotong University Medicine (Grant No. YXJLRH2022095).
AcknowledgmentsThe authors express their gratitude to the Dermatology Department at the Second Affiliated Hospital of Xi’an Jiaotong University.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s noteAll claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary materialThe Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1458438/full#supplementary-material
ReferencesAltunay, I. K., Özkur, E., Dalgard, F. J., Gieler, U., Tomas Aragones, L., Lien, L., et al. (2020). Psychosocial aspects of adult acne: data from 13 European countries. Acta Derm. Venereol. 100 (4), adv00051. doi:10.2340/00015555-3409
PubMed Abstract | CrossRef Full Text | Google Scholar
Ang, P. S., Chen, Z., Chan, C. L., and Tai, B. C. (2016). Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin. Drug Saf. 15 (5), 583–590. doi:10.1517/14740338.2016.1167184
PubMed Abstract | CrossRef Full Text | Google Scholar
Brightling, C. E., Chanez, P., Leigh, R., O'Byrne, P. M., Korn, S., She, D., et al. (2015). Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 3 (9), 692–701. doi:10.1016/s2213-2600(15)00197-6
PubMed Abstract | CrossRef Full Text | Google Scholar
Busse, W. W., Brusselle, G. G., Korn, S., Kuna, P., Magnan, A., Cohen, D., et al. (2019). Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur. Respir. J. 53 (2), 1800948. doi:10.1183/13993003.00948-2018
PubMed Abstract | CrossRef Full Text | Google Scholar
Clemmesen, M. E. R., Gren, S. T., Frøstrup, A. G., Thomsen, S. F., Egeberg, A., and Thein, D. (2024). Psychosocial and mental impact of alopecia areata: analysis of the Danish Skin Cohort. J. Eur. Acad. Dermatol Venereol. doi:10.1111/jdv.20211
CrossRef Full Text | Google Scholar
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10 (6), 483–486. doi:10.1002/pds.677
PubMed Abstract | CrossRef Full Text | Google Scholar
Gandhi, K., Shy, M. E., Ray, M., Fridman, M., Vaghela, S., and Mostaghimi, A. (2023). The association of alopecia areata-related emotional symptoms with work productivity and daily activity among patients with alopecia areata. Dermatol Ther. (Heidelb) 13 (1), 285–298. doi:10.1007/s13555-022-00864-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Gorbatenko-Roth, K., Wood, S., Johnson, M., Wallander, I., Nugent, J., and Hordinsky, M. (2023). Beyond health-related quality of life: initial psychometric validation of a new scale for addressing the gap in assessing the full range of alopecia areata psychosocial burden. Br. J. Dermatol 189 (1), 71–79. doi:10.1093/bjd/ljad054
PubMed Abstract | CrossRef Full Text | Google Scholar
Gutermuth, J., Pink, A. E., Worm, M., Soldbro, L., Bjerregård Øland, C., and Weidinger, S. (2022). Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br. J. Dermatol 186 (3), 440–452. doi:10.1111/bjd.20832
PubMed Abstract | CrossRef Full Text | Google Scholar
Iwase, R., Ishiguro, T., Fujita, K., Ishibashi, S., and Yokota, T. (2020). Dupilumab for atopic dermatitis, a possible risk factor of juvenile ischemic stroke: a case report. J. Stroke Cerebrovasc. Dis. 29 (6), 104763. doi:10.1016/j.jstrokecerebrovasdis.2020.104763
PubMed Abstract | CrossRef Full Text | Google Scholar
Jain, D., Sharma, G., and Kumar, A. (2023). Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs). Expert Opin. Drug Saf. 22 (8), 741–752. doi:10.1080/14740338.2023.2189698
PubMed Abstract | CrossRef Full Text | Google Scholar
Lugović-Mihić, L., Meštrović-Štefekov, J., Potočnjak, I., Cindrić, T., Ilić, I., Lovrić, I., et al. (2023). Atopic dermatitis: disease features, therapeutic options, and a multidisciplinary approach. Life (Basel) 13 (6), 1419. doi:10.3390/life13061419
PubMed Abstract | CrossRef Full Text | Google Scholar
Müller, S., Maintz, L., and Bieber, T. (2024). Treatment of atopic dermatitis: recently approved drugs and advanced clinical development programs. Allergy 79 (6), 1501–1515. doi:10.1111/all.16009
PubMed Abstract | CrossRef Full Text | Google Scholar
Muntyanu, A., Gabrielli, S., Donovan, J., Gooderham, M., Guenther, L., Hanna, S., et al. (2023). The burden of alopecia areata: a scoping review focusing on quality of life, mental health and work productivity. J. Eur. Acad. Dermatol Venereol. 37, 1490–1520. doi:10.1111/jdv.18926
CrossRef Full Text | Google Scholar
Popovic, B., Breed, J., Rees, D. G., Gardener, M. J., Vinall, L. M., Kemp, B., et al. (2017). Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13rα1 and IL-13rα2. J. Mol. Biol. 429 (2), 208–219. doi:10.1016/j.jmb.2016.12.005
PubMed Abstract | CrossRef Full Text | Google Scholar
Rivkees, S. A., and Szarfman, A. (2010). Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J. Clin. Endocrinol. Metab. 95 (7), 3260–3267. doi:10.1210/jc.2009-2546
PubMed Abstract | CrossRef Full Text | Google Scholar
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13 (8), 519–523. doi:10.1002/pds.1001
PubMed Abstract | CrossRef Full Text | Google Scholar
Sharma, A., and Kumar, A. (2022). Identification of novel signal of clobazam-associated drug reaction with eosinophilia and systemic symptoms syndrome: a disproportionality analysis. Acta Neurol. Scand. 146 (5), 623–627. doi:10.1111/ane.13690
PubMed Abstract | CrossRef Full Text | Google Scholar
Sharma, A., Roy, S., Sharma, R., and Kumar, A. (2023). Association of antiviral drugs and their possible mechanisms with DRESS syndrome using data mining algorithms. J. Med. Virol. 95 (3), e28671. doi:10.1002/jmv.28671
PubMed Abstract | CrossRef Full Text | Google Scholar
Shu, Y., Ding, Y., Liu, Y., Wu, P., He, X., and Zhang, Q. (2022a). Post-marketing safety concerns with secukinumab: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 13, 862508. doi:10.3389/fphar.2022.862508
PubMed Abstract | CrossRef Full Text | Google Scholar
Shu, Y., He, X., Liu, Y., Wu, P., and Zhang, Q. (2022b). A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Clin. Epidemiol. 14, 789–802. doi:10.2147/clep.S365513
PubMed Abstract | CrossRef Full Text | Google Scholar
Silverberg, J. I., Toth, D., Bieber, T., Alexis, A. F., Elewski, B. E., Pink, A. E., et al. (2021). Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Dermatol 184 (3), 450–463. doi:10.1111/bjd.19573
PubMed Abstract | CrossRef Full Text | Google Scholar
Wollenberg, A., Beck, L. A., de Bruin Weller, M., Simpson, E. L., Imafuku, S., Boguniewicz, M., et al. (2022). Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br. J. Dermatol 186 (3), 453–465. doi:10.1111/bjd.20810
PubMed Abstract | CrossRef Full Text | Google Scholar
Wollenberg, A., Blauvelt, A., Guttman-Yassky, E., Worm, M., Lynde, C., Lacour, J. P., et al. (2021). Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol 184 (3), 437–449. doi:10.1111/bjd.19574
PubMed Abstract | CrossRef Full Text | Google Scholar
Wollenberg, A., Howell, M. D., Guttman-Yassky, E., Silverberg, J. I., Kell, C., Ranade, K., et al. (2019). Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 143 (1), 135–141. doi:10.1016/j.jaci.2018.05.029
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhang, Y., Jing, D., Cheng, J., Chen, X., Shen, M., and Liu, H. (2022). The efficacy and safety of IL-13 inhibitors in atopic dermatitis: a systematic review and meta-analysis. Front. Immunol. 13, 923362. doi:10.3389/fimmu.2022.923362
Comments (0)