Panax ginseng C.A.Mey. [Araliaceae, Ginseng radix et rhizoma], as a traditional botanical drug with a long history, has occupied a pivotal position in Chinese traditional medicine since ancient times. P. ginseng has a slightly bitter and warm taste, serving as a great tonic that can boost energy, restore the pulse, nourish the spleen and lungs, replenish blood, calm nerves, and improve intelligence (National Pharmacopoeia Commission, 2020). With the development of modern science and technology, more medicinal values of P. ginseng have been gradually explored by researchers around the world, and its application fields have been expanded from food and health products to the medical field (Mancuso and Santangelo, 2017). More than 300 active metabolites, including polysaccharides, ginseng peptides, ginsenosides, flavonoids (Liu et al., 2020), volatile oils, organic acids, alkaloids, trace elements, and vitamins, have been identified (Su et al., 2023). The medicinal value of P. ginseng is not only reflected in its polysaccharides, ginsenosides and other metabolites, volatile oil as one of the metabolites. Although it only accounts for about 0.02%–2.5% (Peng et al., 2017; Sun et al., 1993), its biological activity cannot be ignored. P. ginseng volatile oil (GVO) has shown significant efficacy in cardiovascular protection, antimicrobial, anti-aging, anti-platelet aggregation, anti-inflammatory, nutritional support, and neurocellular protection. The diversity of its chemical metabolites and the potential of its pharmacological activities provide a wide scope for future developmental studies.
In recent years, relatively little research has been conducted on the volatile oil of ginseng, and limited data are available for reference. This has prompted us to conduct a more comprehensive and in-depth exploration of it. In this paper, we will analyze the chemical composition of GVO, discuss the volatile oil metabolites extracted from different parts of P. ginseng and their pharmacological effects, and also investigate the effects of different regions and years of growth on the volatile oil content of P. ginseng. The summary was further refined with the help of the newidea.ai (https://www.newidea.ai/home). In terms of the extraction process, this paper will compare the advantages and disadvantages of traditional and modern methods and explore their effects on the composition and medicinal effects of volatile oils. In terms of pharmacological effects and mechanisms of action, this paper will detail the effects of GVO on the cardiovascular system, anti-inflammatory, antibacterial, anticancer, etc., and explore its potential molecular targets and mechanisms of action (Figure 1). In summary, this paper will provide a comprehensive theoretical basis for the research and application of GVO and point out the direction for future development and utilization. Through in-depth exploration of the chemical composition, pharmacological activity and mechanism of action of GVO, we expect to be able to provide scientific support for the in-depth development and clinical application of P. ginseng resources.
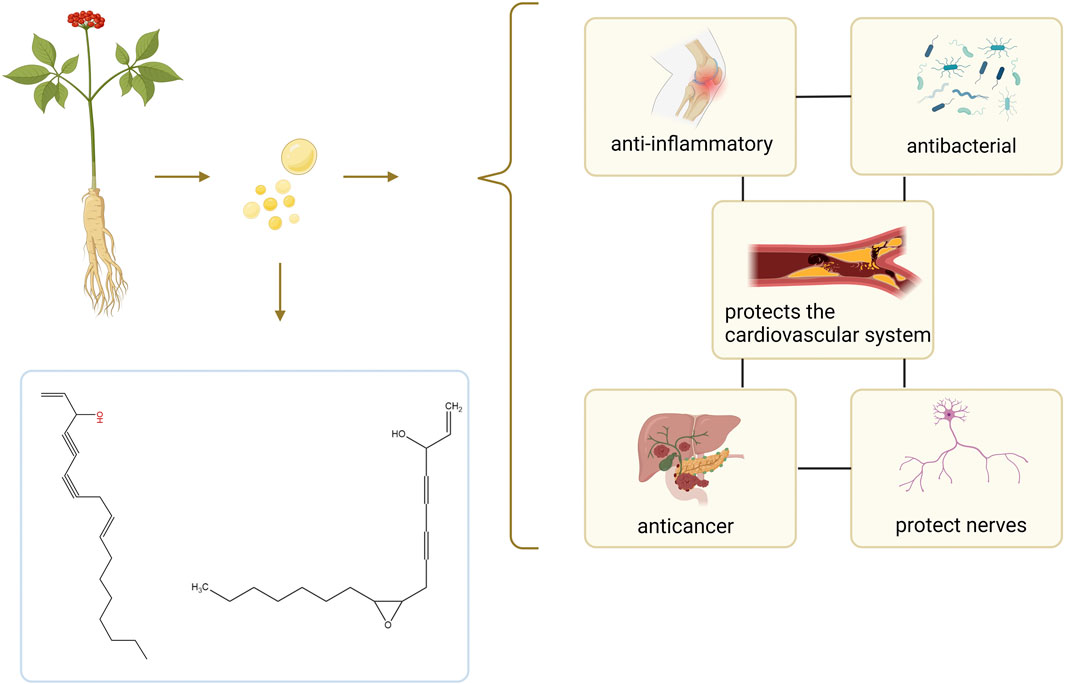
Figure 1. Summary of the composition and effect of GVO. GVO has a special aroma, and its main metabolites are Linoleic acid, panaxynol, panaxydol and so on. It has anti-inflammatory, antioxidant, anti-aging, anti-cancer, neuroprotective, and fatigue-relieving properties.
2 The phytochemical composition of GVOGVO possesses a distinct aroma. The P. ginseng aroma developed through organic culture method (OCM) and GAP method exhibited the highest levels of beet saponin and aromatic alkene, which are recognized as key metabolites of the P. ginseng aroma (Lee et al., 2012). In research, GC-MS is commonly utilized to assess the composition and quality of volatile oils (Daferera et al., 2000). Through this method, modern scholars have identified 369 metabolites in GVO, comprising 154 hydrocarbons, 35 ketones, two aldehydes, 55 esters, 37 alcohols, 12 acids, 22 nitrogen-containing metabolites, and 52 other metabolites (Qiu et al., 2008), as shown in Figure 2; Table 1. Among the volatile metabolites of P. ginseng, closely related to the aroma properties of the plant are sesquiterpenoids, accounting for about 40% of the volatile oil, followed by panaxynol and panaxydol (Cho et al., 2012). Currently, over 20 types of polyacetylene derivatives have been isolated from P. ginseng (Yeo et al., 2017), including panaxynol, panaxydol, panaxydiol, panaxytriol, panaxacol, panaxyne epoxide, ginsenoyne A− K. Recently, new polyacetylenes metabolites with carbonyl group replacing the hydroxyl group had been isolated such as 9,10-epoxyheptadecan-4,6-diyn-3-one, one-ethoxy-9,10-epoxyheptadecan-4,6-diyn-3-one and 9,10-epoxy-16-heptadecan-4,6-diyn-3-one.
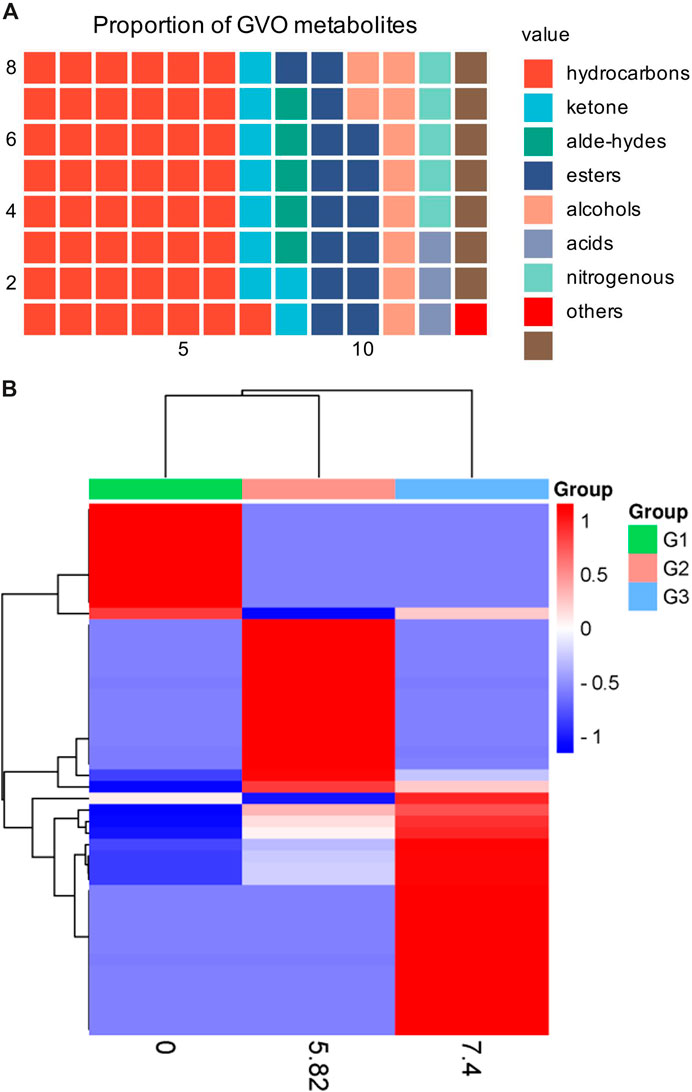
Figure 2. The main metabolites of GVO and its differences under diverse processing conditions. (A) Proportion of different types of metabolites of GVO. (B) Heat map of chemical composition differences among fresh ginseng G1, white ginseng G2, and red ginseng G3.
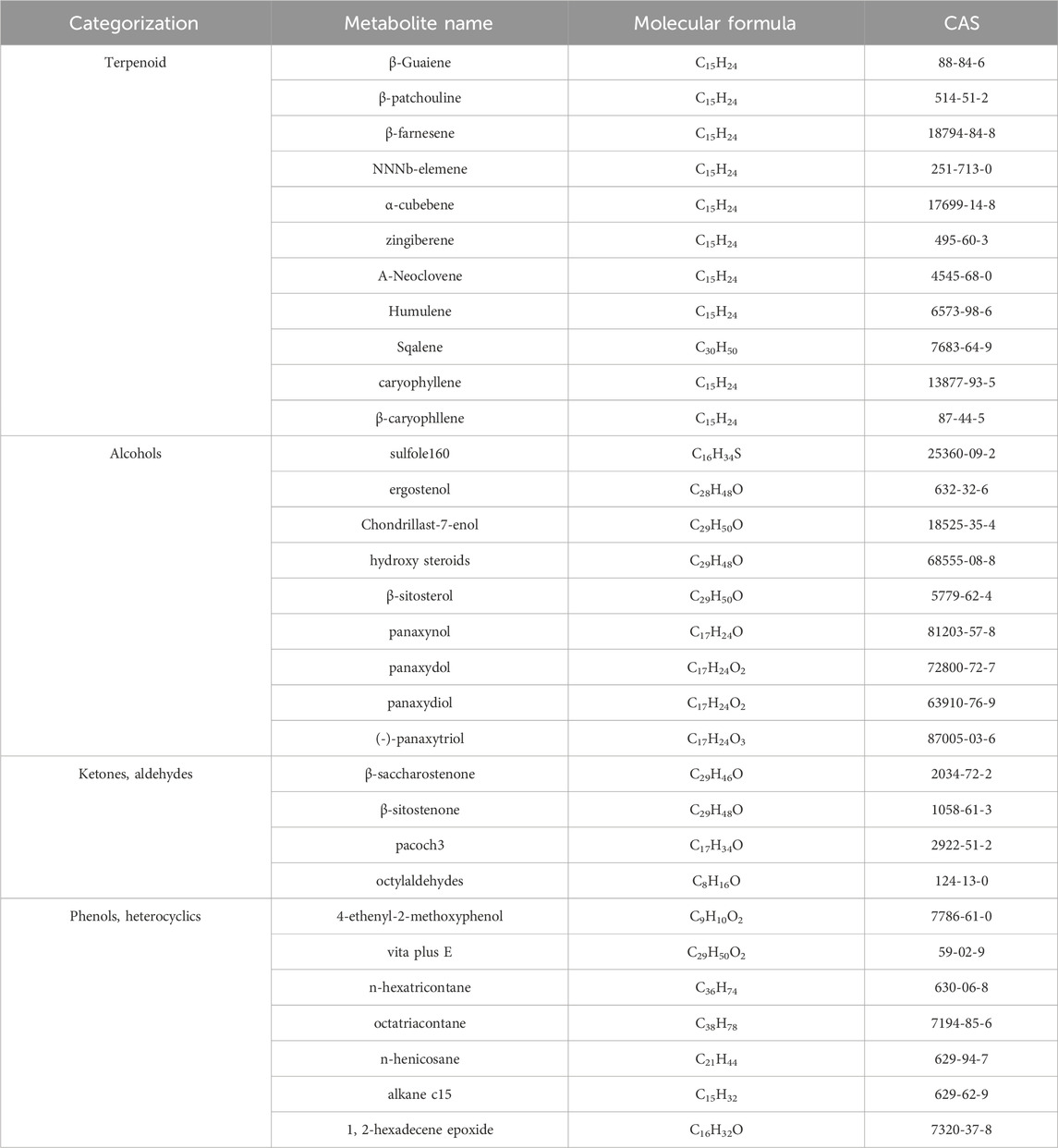
Table 1. Partial metabolites of GVO.
2.1 A chemical analysis of the volatile oils extracted from different parts of the P. ginseng plant2.1.1 Volatile oil metabolites in flower budsThe volatile oil content of P. ginseng varies across different parts of the plant. Although P. ginseng flowers (GFs) buds are not recorded in the Chinese Pharmacopoeia (2020 edition), GFs are also non-traditional medicinal parts with anti-fatigue and immune-enhancing properties. Studies have shown that the composition of volatile oils in P. ginseng flowers generally remains consistent over time (Mao et al., 1989). The volatile oil extracted from it is a light yellow transparent oily liquid with a yield of about 0.2%. After identification, 23 chemical metabolites were identified from 51 chromatographic peaks, including 10 sesquiterpenes, eight alkanes, 2 esters, and one ketone, accounting for 43.5%, 34.8%, 8.7%, 8.796, and 4.3% of the total volatile oils, respectively (Figure 3). Among the sesquiterpenes, α-solaninene (Figure 3A), α-sandalpinene, β-sandalene and (3Z,6E)-α-farnesene were discovered for the first time.
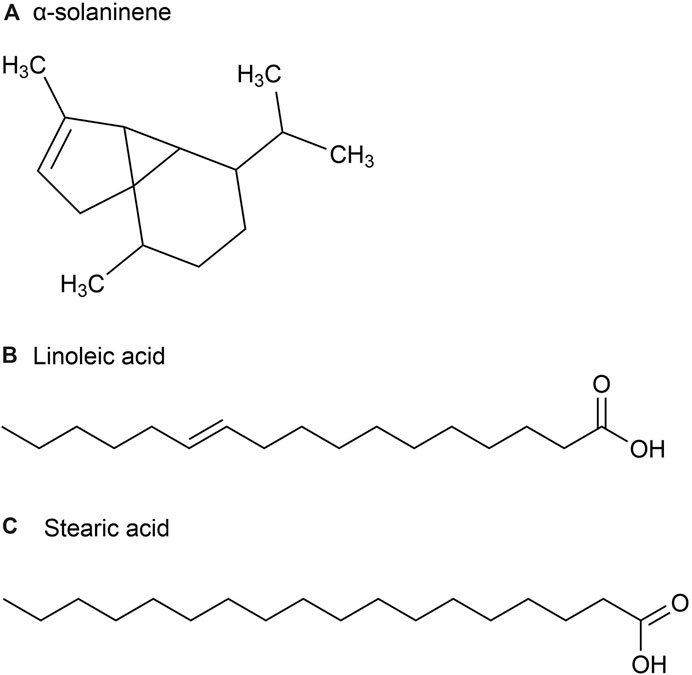
Figure 3. The main metabolites of GVO in flower buds. (A) α-solaninene. (B) Linoleic acid. (C) Stearic acid.
Furthermore, some scholars have used GC-MS analysis to identify 45 chemical metabolites in the volatile oil of P. ginseng flowers (Ma et al., 1992). Among them, the highest content is Linoleic acid (37.06%) (Figure 3B), followed by Stearic acid (17.77%) (Figure 3C). Other main metabolites include aldehydes, enals, unsaturated alcohols, higher alkanes, alkynes, etc. Distribution density and accumulation of oil cells affect volatile oil content. Therefore, P. ginseng flowers may contain volatile oils that are closely related to oil cell growth and development (McAdam et al., 2020). As P. ginseng blooms after 3 years, its early accumulation of substances is deeper, providing more energy and nutrients to oil cells. Consequently, the volatile oil content is the highest in three-year-old P. ginseng flowers (Du et al., 2023).
2.1.2 Volatile oil metabolites in stems and leavesTraditionally, P. ginseng leaves have been utilized in China as a medicine for treating diseases. In comparison to P. ginseng roots, its leaves have a shorter growth period and lower cost, making them both economically and medicinally valuable. However, little attention has been given to the chemical composition of the volatile oil in P. ginseng leaves. In a study, P. ginseng leaves and stems were extracted, yielding a black-green crystalline volatile oil of 0.14% (Liu et al., 2002).
A total of 54 metabolites were identified, predominantly consisting of aliphatic (69.0%), terpenoids (21.5%) and aromatic (2.4%). The major metabolites identified in these parts include Palmitic acid (36.1%) (Figure 4A), followed by (E)-β-farnesene (15.4%), Linoleic acid (9.8%) (Figure 4B), phytol (5.6%) (Figure 4C) and methyl hexadecoate (2.9%) (Figure 4D). Sesquiterpene hydrocarbons accounted for 20.3%, while oxidized sesquiterpene hydrocarbons accounted for 0.6%, and monoterpene hydrocarbons accounted for 0.6% of terpenoids (Jiang et al., 2014).
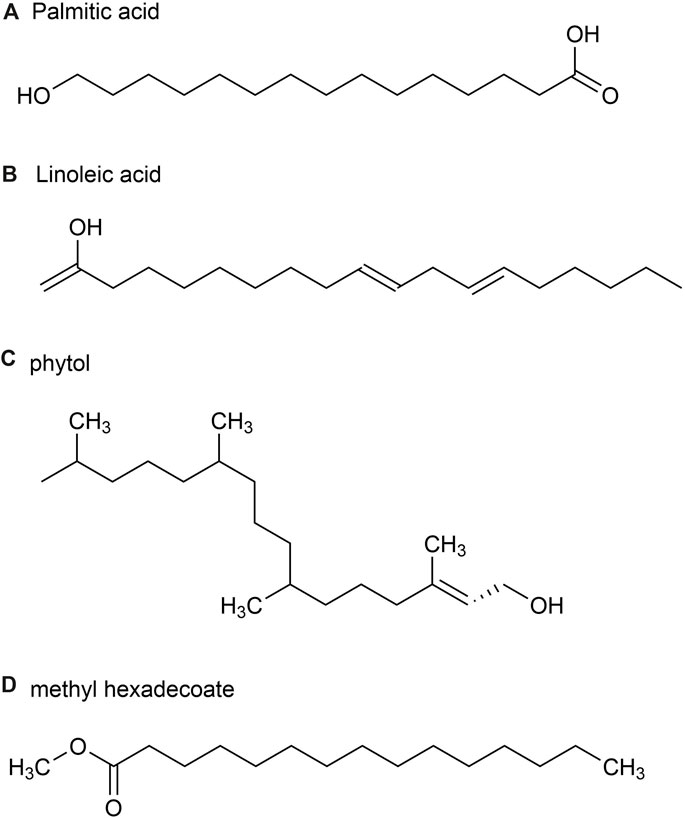
Figure 4. The main metabolites of GVO in stems and leaves. (A) palmitic acid. (B) Linoleic acid. (C) phytol. (D) methyl hexadecoate.
2.1.3 Volatile oil metabolites in fruitsP. ginseng fruit is the dried ripe fruit of P. ginseng. Its chemical metabolites include ginsenoside, volatile oil, carbohydrate and sugar, amino acid and allaloids, vitamins and minerals. A total of 23 volatile metabolites, mainly composed of sesquiterpenes, have been identified from P. ginseng fruits of three different colors, red fruit, yellow fruit, and orange fruit, such as (E)-β-farnesene (Figure 5A), β-Elemene (Figure 5B), Santene, Cedarene (Figure 5C), and α-neoclovene (Figure 5D). The total sesquiterpene content of red fruits is the highest, followed by orange and yellow fruits, with significant differences between samples. Yellow fruits contain a significant amount of δ-selinene (Figure 5E), β-caryophyllene, α-farnesene ginsenosol and cadinol (Figure 5F). As a consequence, P. ginseng fruit has different volatile (Cui et al., 2020).
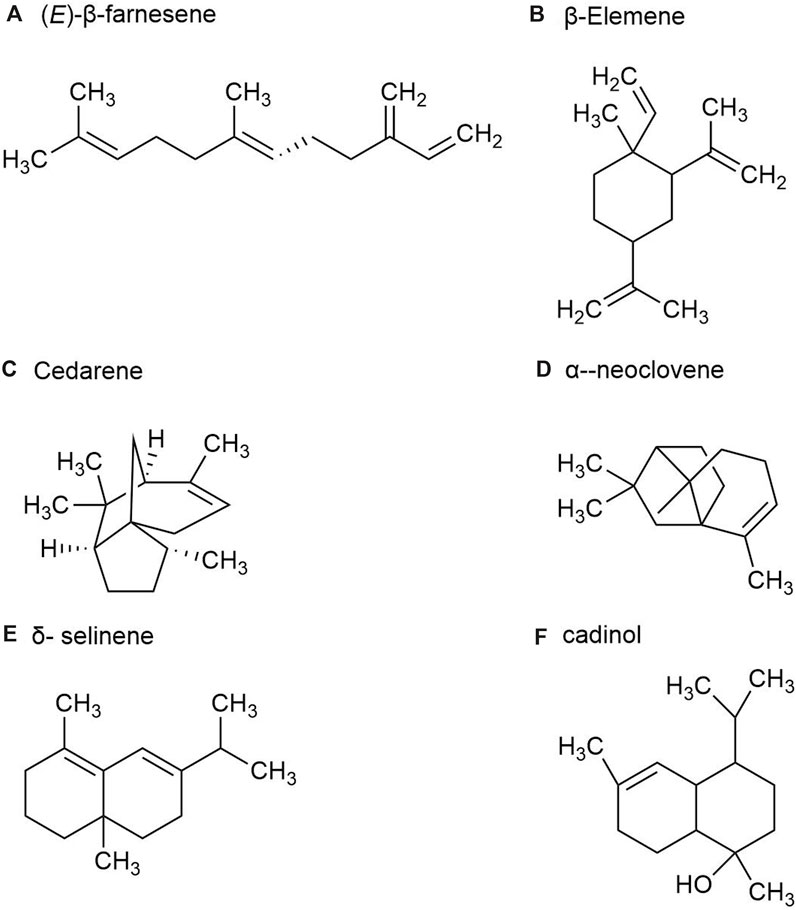
Figure 5. The main metabolites of GVO in fruits. (A) (E)-β-farnesene. (B) β-Elemene. (C) Cedarene. (D) α-neoclovene. (E) δ-selinene. (F) cadinol.
2.1.4 Volatile oil metabolites in rhizomesThere are more than 40 kinds of chemical metabolites in the volatile oil of P. ginseng root, mainly including esters, monoterpenes, alkanes, and sesquiterpenes. As part of the sesquiterpene family, sesquiterpenoids are characteristic metabolites of GVO. Such as β-Ginsenene, (−)-α- Gurjunene (Figure 6A), β-Elemene, β-Caryophyllene (Figure 6B), β-New clove tricycline (molecular formula C15H24) and sesquiterpene oxygen-containing metabolites (mainly referring to alcohols such as spartanol, ginsenosol, and -(−)globulol (Figure 6C)) α-Juniperol, etc., with a molecular formula of C15H24O) (Richter et al., 2005; Ding, 2008). It was found that the content of total volatile oil in roots increased with the growth age of P. ginseng. A study was conducted by steam distillation to extract the volatile oil content in P. ginseng reeds, and the yield was 0.35%. The main differences with P. ginseng root were palmitic acid, 2,6-ditert-butyl-4-methylphenol (Figure 6D) and methyl octadenoate, with the contents of 2.08%, 1.80% and 1.44%, respectively (Zheng et al., 1989).
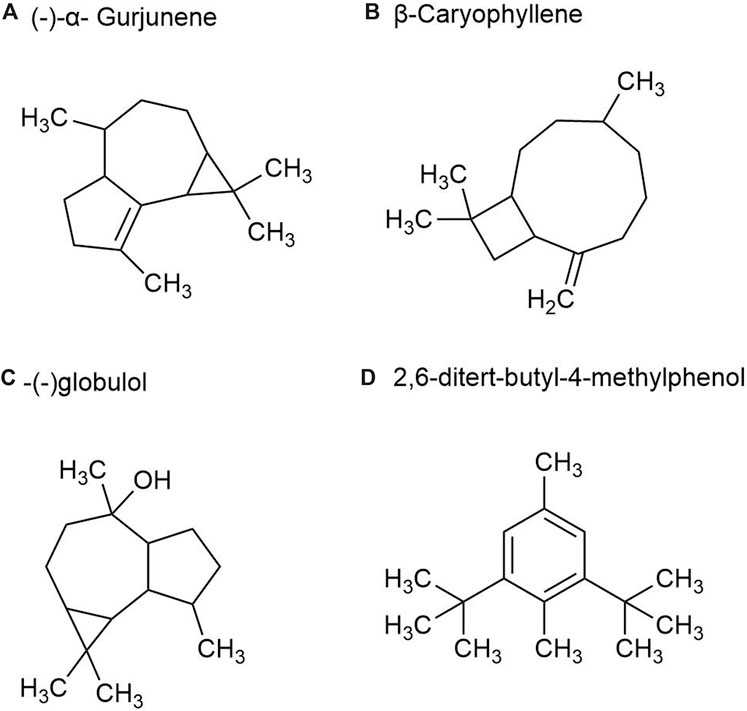
Figure 6. The main metabolites of GVO in rhizomes. (A) (-)-α- Gurjunene. (B) β-Caryophyllene. (C) -(-)globulol. (D) 2,6-ditert-butyl-4-methylphenol.
The composition of volatile oils from different parts of P. ginseng varies, as shown in Figure 7. Sesquiterpenes were the most abundant metabolites in flowers, followed by alkanes and esters. The stems and leaves contain sesquiterpenes, aromatic metabolites. The fruit of P. ginseng has the highest percentage of sesquiterpene content compared to other parts of the plant. In P. ginseng root, the main metabolites are sesquiterpenes and alkanes. In addition to this, there is also oil in P. ginseng seeds. P. ginseng seed oil is mainly composed of non-volatile fatty acids, followed by phenolic compounds (Zhu et al., 2010; Lee et al., 2013)
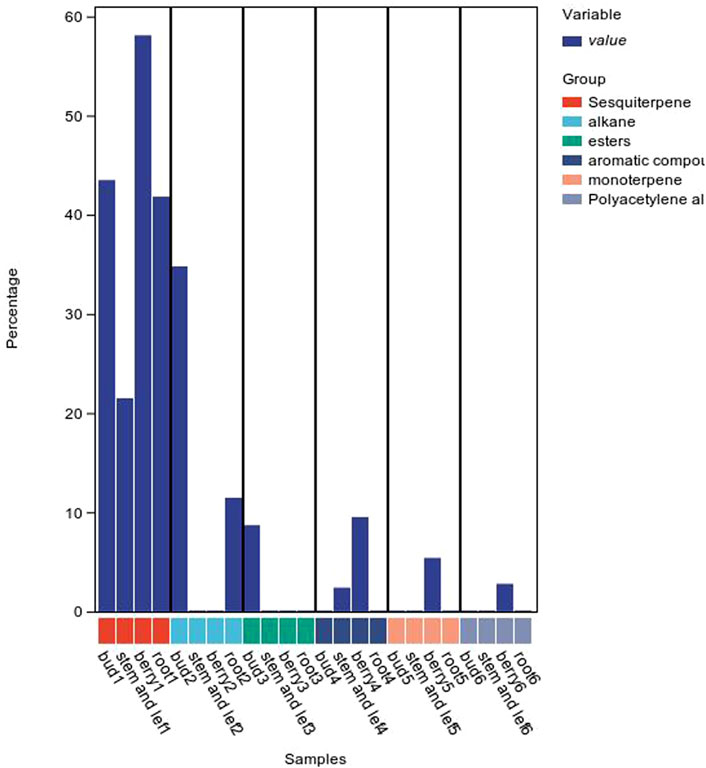
Figure 7. Volatile oil content of P. ginseng in different parts.
2.2 Effects of different regions on the volatile oil content of P. ginsengThe volatile oil composition and content of P. ginseng varied in different regions and years of growth, as shown in Figure 8. A study was conducted to compare and summarize the quality and yield of GVO from several counties under the provinces of Jilin, Liaoning, and Heilongjiang with those of Korean ginseng. Among the P. ginsengs of different ages and origins, the one with the highest volatile oil yield was the four-year-old Antu P. ginseng, and the one with the lowest yield was the six-year-old Jian P. ginseng. The mean value of the volatile oil yield of P. ginseng roots from all origins was 0.056%, with an RSD of 26%, indicating that the volatile oil content of P. ginseng differed significantly among different origins and ages (Wang, 2016).
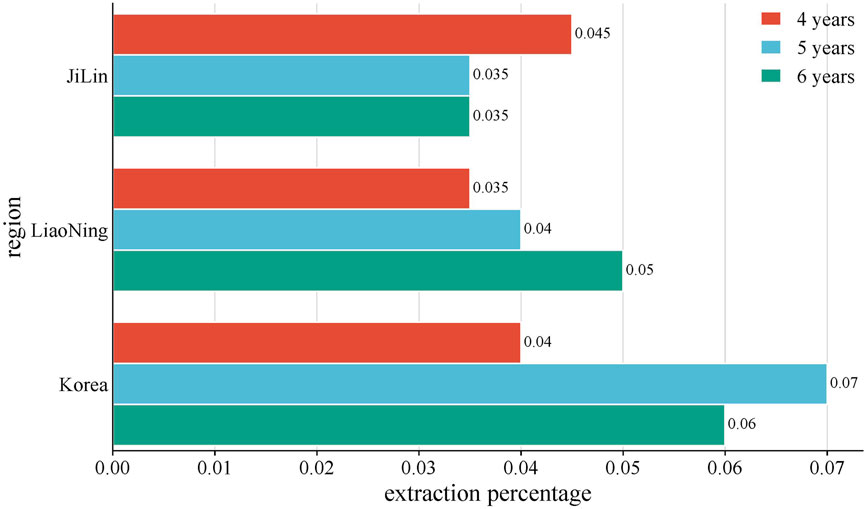
Figure 8. Average yield of GVO by region. The average volatile oil yield of P. ginseng from Jilin, Liaoning, and South Korea in China was compared with. It was found that the volatile oil content of four-year-old P. ginseng: Jilin Province > Liaoning Province > Korea. Five-year-old P. ginseng: Korea > Liaoning Province > Jilin Province. Six-year-old P. ginseng: Liaoning Province > Korea > Jilin Province.
Different growth forms of P. ginseng have varying levels of volatile oil content. The volatile oils of cultivated P. ginseng (CG), transplanted P. ginseng (TG) and mountain cultivated P. ginseng (MCG) were extracted by headspace solid-phase microextraction-gas chromatography-mass spectrometry, followed by chromatographic identification using n-alkane standard (C7-C30). Calculating and comparing the aldehydes, terpenes, alcohols, alkynes, esters, and other metabolites of three types of P. ginseng, it was found that the content of terpenoids was the highest, with CG (85.91%), MCG (90.27%), and TG (76.89%), respectively. However, a difference in alkyl alcohol content between P. ginseng samples of different origins was not statistically significant (Gu et al., 2022).
2.3 Effect of concoction on the volatile oil composition of P. ginsengWhen P. ginseng is steamed and dried, it is produced as red ginseng. Research has indicated that the conversion of P. ginseng into red ginseng leads to a loss in total volatile oil content ranging from 63.89% to 74.54%, averaging 69.50% (Wu et al., 1992). In addition, the composition of volatile oil is altered during this process, as depicted in Figure 2B. The transformation of P. ginseng into red ginseng results in a change in the composition of GVO. The main metabolites of red ginseng oil (RGO) include linoleic acid, palmitic acid, β-sitosterol, γ-sitosterol, and stigmasterol, which are also present in GVO. The relative content of C4-C6 metabolites in red ginseng and fresh ginseng differs significantly, with fresh ginseng containing 1.04% of C4-C6 metabolites compared to red ginseng. Fresh ginseng contains three C10 monoterpenes, while red ginseng contains only one. The content of soy sterols and β-sitosterol also differed in red ginseng and P. ginseng. Notably, the content of stigmasterol in five-year-old and six-year-old red ginseng was reported to be 23.84 mg/g and 27.46 mg/g, respectively. The content of beta-sitosterol in five and six-year-old P. ginseng was 72.58 mg/g and 82.14 mg/g (Lee et al., 2018) respectively. In addition, a study comparing the volatile characteristics of fresh, white and red ginseng, found that fresh P. ginseng had a stronger odor than red ginseng (Abd El-Aty et al., 2008). The main functional groups identified in white and red ginseng were alcohols, ketones, esters, and phenols, with acids being found only in fresh P. ginseng. Therefore, it can be hypothesized that during the processing of fresh ginseng, many volatile metabolites may disappear or increase (Cho, 2015).
For different concoctions, the content of stigmasterol (metabolite 1) and beta-sitosterol (metabolite 2) in P. ginseng varied greatly from year to year. Of the three concocted forms, the total metabolite content of red ginseng was least affected by year. White P. ginseng showed the greatest variation in content and had the highest levels of metabolites in the six-year-old. So when it comes to experiments related to P. ginseng phytosterols, researchers need to choose according to their own experimental requirements (Lee et al., 2018).
Although there are differences in the composition of volatile oils derived from red ginseng and P. ginseng, their pharmacological effects, mechanism of action, targets and pathways are comparable. RGO has been found to possess antitumor activity (Lee et al., 2010). It inhibits tumor transformation and blocks the activation of NF-kB, AP-1, and MAPK, as well as the expression of COX-2 (Truong et al., 2018). This anticancer pathway is similar to that of GVO. β-sitosterol and linoleic acid (Yasuda et al., 2009) present in RGO have been identified as effective substances with anti-tumor and neuroprotective properties (Lee et al., 2017). β-Sitosterol promotes cell cycle arrest and apoptosis in breast cancer cells (Vundru et al., 2013), prostate cancer cells (von Holtz et al., 1998), and inhibits proliferation of human gastric adenocarcinoma cells and xenograft tumors. Furthermore, RGO also has anti-inflammatory effects (Bak et al., 2012a), which can significantly reduce the serum levels of NO, IL-6 and TNF-a in mice, as well as the expression of colon inflammation markers iNOS, COX-2, IL-6, IL-1β and TNF-α (Truong et al., 2019). Similarly, GVO can also reduce the aforementioned inflammatory factors in the serum to achieve anti-inflammatory effects.
Red ginseng, exhibits higher antioxidant activity due to the increase in phenolic metabolites induced by steam during the preparation process (Kang et al., 2006). RGO can effectively inhibit DPPH and ABTS free radicals. It may also significantly reduce the levels of liver enzymes (ALT and AST) in the serum of mice, increase the levels of antioxidant enzymes (SOD and CAT), reduce the content of DNA oxidation products (8-OHdG) (Ullah et al., 2021), and protect the liver from oxidative stress. Moreover, red ginseng oil also directly scavenges ROS (Meerson et al., 1982), inhibits lipid peroxidation, and protects cells from oxidative damage by inhibiting the MAPK signaling pathway to induce the expression of cellular antioxidant enzyme activity (Abe and Berk, 1998). In addition, RGO also has antibacterial effects (Reyes et al., 2017) and has the ability to control acne. It promotes anti-melanin production (Saba et al., 2020), hair growth, and protects the skin from UVC radiation (Truong et al., 2021). GVO is similar to red GVO chemical composition. However, during the processing of red ginseng, some metabolites are lost while new substances are produced. Both types of volatile oil exhibit similar pharmacological effects, but further comparative studies are necessary to determine which one yields superior results.
2.4 Effects of different growth years on the volatile oil content of P. ginsengThe volatile oil content of P. ginseng varies with the plant’s age, generally showing an increasing trend as the ages. The older the P. ginseng, the better its quality, mainly due to the accumulation of active metabolites with age. Research has found that the relative abundance of a-cadinol, a-bisabolol, thujob-sene, and n-hexadecanoic acids in volatile oils increases most significantly. By comparing the relative amounts of these metabolites, the quality of GVO can be evaluated (Qiu et al., 2008).
Principal metabolite analysis (PCA) was performed on the volatile oil of P. ginseng during the third, fifth and eighth year growth periods, and it was found that there were significant differences in the volatile oil of different years. In particular, the samples of groups 7, 8 and 9 had obvious dispersion compared with other groups, which proved that there was a significant difference in the composition of eight-year-old P. ginseng compared to samples of other ages. The spots on samples 1, 2, and three are located in smaller areas, indicating that the chemical composition differences of the samples over the past 3 years are relatively small.
Samples 1, 2, and three all have spots located in smaller areas, indicating that there is very little difference in chemical composition between the samples over the course of the past 3 years.
3 Extraction process of GVOThe volatile oil of P. ginseng is composed of various metabolites with low content, solubility, and boiling points, as well as highly unstable properties. Therefore, the efficiency and rationality of the extraction method are crucial. Volatile oil extraction methods can be classified into traditional and innovative methods. Traditional methods include steam distillation, impregnation, infiltration, and reflux extraction. With technological advancements, new methods such as ultrasonic extraction, microwave extraction, semi-biomimetic extraction, and solid phase microextraction have been developed (Table 2). Among the available techniques, supercritical fluid extraction technology offers a higher extraction rate and less pollution, although it is not suitable for large-scale production. The composition of volatile oils from traditional Chinese medicine can vary based on the extraction method. The most appropriate extraction method should be chosen based on the specific circumstances.
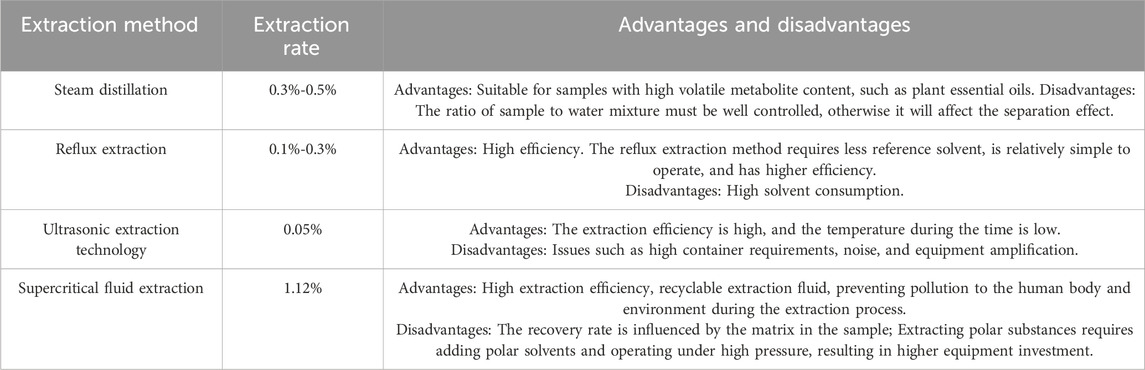
Table 2. Comparison of different extraction processes for GVO.
3.1 Traditional extraction methods3.1.1 solvent extraction (SO)Solvent extraction is a common method used in practice. Based on the solubility properties of GVO, it can be extracted using the soxhlet extraction method or cold immersion method with organic solvents like petroleum ether (30–60°C), ether, or carbon tetrachloride. The working principle involves the solvent penetrating the cell membrane of botanical drugs, dissolving soluble substances, creating a concentration difference between the inside and outside of the cells, and allowing the solute to permeate out of the cell membrane (Kuang, 2011). After vacuum distillation to eliminate organic solvents, the extract is obtained. Subsequently, hot ethanol is employed to dissolve the extract, which is then cooled, filtered to remove impurities, and the ethanol is reclaimed to obtain clean oil.
The extract can also be re-distilled to acquire a purer essential oil. Studies have explored the extraction of volatile oil using various solvents. Research indicates that the extraction process utilizing water as the solvent can yield the highest levels of phenolic substances and flavonoids (El et al., 2011). This extraction method is straightforward, practical, and enables the extraction of the natural metabolites of plant volatile oil. However, extracting essential oils through leaching with organic solvents is more intricate and often leads to significant solvent residue problems.
3.1.2 Steam distillation methodResearch has shown that steam distillation is the most efficient method for obtaining volatile oils, with an extraction efficiency of 93% according to studies (Aziz et al., 2018). The volatile oil is not mixed with water. When the combined vapor pressures of the volatile oil and water equal the atmospheric pressure, the solution boils. If further heated, the volatile oil can be distilled out with water vapor.
During extraction, the crude powder of the raw material can be soaked in water in a still and then directly heated and distilled, or the raw material can be placed on a perforated partition plate net. As the steam generated by heating the water passes through the raw material, the volatile oil is heated and distilled out simultaneously with the water vapor. Collect distillate, cool it and separate the oil layer (Pei, 2016). This method for extracting GVO offers advantages such as simple equipment, easy operation, low cost, large yield, and high recovery rate of volatile oil, However, it should be noted that the raw materials are prone to coking due to the intense heat. Additionally, the heating of volatile oil during the extraction process can lead to chemical reactions such as molecular isomerization, which can affect the composition and reduce the value of the volatile oil (Ma et al., 1985).
Although traditional extraction methods are commonly used in production, they come with some inherent drawbacks. Apart from long extraction times, they necessitate a large amount of solvent and energy. Prolonged contact with hot water or steam can degrade certain metabolites and hydrolyze them. Furthermore, the lack of adjustable parameters in these methods makes it challenging to control the process selectivity and essential oil concentration (Yang et al., 2014).
3.2 Modern extraction methods3.2.1 Supercritical fluid extraction (SFE)For the extraction of plant volatile oils, supercritical fluid extraction (SFE) is a relatively new and efficient method. SFE is faster, more convenient, and more selective than traditional extraction methods, with higher extraction rates and lower temperatures. In a study, the process of extracting volatile oil from supercritical CO2 was optimized by using raw sun-dried P. ginseng as raw material. Response surface analysis was employed to determine the optimal extraction conditions (Cui et al., 2016). The results indicated that an extraction pressure of 38 MPa, an extraction temperature of 55°C, a static extraction time of 2 h, and a dynamic extraction time of 1 h resulted in an extraction rate of 1.12%. This method allows for the simultaneous separation of high and low boiling point substances, resulting in a product that is richer in oil metabolites. In addition, it enables the extraction of both volatile and non-volatile GVO, significantly improving the overall yield (Pourmortazavi and Hajimirsadeghi et al., 2007). In the study of P. ginseng seed oil extraction, it was found that supercritical fluid extraction yielded higher oil content compared to compression or solvent extraction. The highest yield of P. ginseng seed oil extracted by supercritical fluid extraction was 17.48% at 500 bar and 65°C (Lee et al., 2013). This technology utilizes CO2 as a supercritical fluid, which prevents the destruction of active metabolites and facilitates the development of new drugs. Furthermore, it reduces labor requirements and the use of organic solvents, thereby reducing pollution from the three wastes, making it a modern technology for the extraction of natural essential oils that is vigorously promoted and widely used.
3.2.2 Microwave-assisted extraction methodMicrowave-assisted water distillation (MAHD), which employs water as a solvent, is a sustainable and eco-friendly approach for extracting volatile oils from plants (Golmakani and Rezaei, 2008). During the extraction process, microwave power, liquid-material ratio, extraction time and other parameters have a significant impact on the extraction efficiency. Compared with traditional extraction methods, MAHD significantly shortens extraction time and improves extraction efficiency of essential oils (Shang et al., 2020). There are studies using this method to extract essential oil and polyphenols from camphor leaves, and the yield of essential oil under optimal conditions is 3.26% ± 0.05%. Microwave radiation has the potential to harm cell membranes through cell expansion, modification of intracellular structures, impairment of oil-rich glands and cells, acceleration of the movement of aqueous solutions, and dispersion of internal metabolites (Chen et al., 2016).
3.2.3 Ultrasound-assisted extraction (UAE)The ultrasonic extraction method is the use of ultrasound cavitation, mechanical effects, and thermal effects to increase the frequency and speed of the molecular movement of substances, to promote contact between the solution and the material, from the target to obtain more metabolites (Raj and Dash, 2020; Yang et al., 2021). It has the advantages of time-saving, energy-saving, and low-temperature extraction is conducive to the protection of active metabolites, it is a rapid and efficient new extraction method.
In one study, raw natural-dried P. ginseng powder was used as raw material and ether as solvent in a soxhlet extractor with ultrasonic cleaner at reflux for 90 min in this method. The ether was recovered to obtain the ether leachate, which was subjected to hydrodistillation to collect the distillate. Extracted with ether 5 times, followed by dehydration with anhydrous sodium sulfate and drying to a constant weight. The content determination results revealed that the volatile oil content obtained from the 90-min extraction using the ultrasonic extraction method was in line with the findings reported in the literature (Song, 1991). In another study, ultrasound-assisted pretreatment extraction (UAPE) was employed to extract essential oils from the peels of Tribute citrus (TC) peels, resulting in significantly higher yields compared to traditional hydrodistillation (HD). It has been demonstrated that ultrasonic extraction has a higher extraction rate compared to the conventional method. However, this technique is not suitable for large-scale production (Li et al., 2022).
4 The pharmacological effects of GVO and its mechanism of actionRecently, the pharmacological properties of P. ginseng have been discovered, revealing its potential in areas such as anti-aging, anti-diabetes, anti-cancer, analgesia, antipyretic, anti-stress, anti-fatigue, sedation, and protein-promoting activities (Zhou et al., 2023). The study mainly focuses on the elaboration of ginsenosides, P. ginseng polysaccharides. At present, it has been discovered that the fat-soluble metabolites of P. ginseng possess anti-inflammatory, antitussive, antihypertensive, anti-fatigue, anti-tumor, cholesterol-lowering, and central-nervous-exciting effects. In addition, it is worth mentioning that the pharmacological effects and chemical composition of P. ginseng can be influenced by various factors, including species, geographic location, cultivation, environment, harvesting, storage, and post-harvest processing.
4.1 Cardiovascular effectsGVO has a beneficial therapeutic effect on cardiovascular diseases. The petroleum ether extract of P. ginseng has been shown to significantly inhibit diacylglycerol acyltransferase (DGAT) (Lee et al., 2004) and acyl-CoA: cholesterol acyltransferase (ACAT) (Rho et al., 2005) in rat liver microsomes. ACAT has been explored as a potential target for drug intervention in hyperlipidemia and atherosclerosis (Chhabria and Mahajan, 2009). The preventive mechanism involves inhibiting ACAT in the intestines, liver, and arteries, thereby reducing plasma total cholesterol and low-density lipoprotein cholesterol levels, preventing cholesterol esterification, and reducing cholesterol deposition in arterial walls (Kwon et al., 1997). Spectral analysis identified the chemical structures of metabolites in the petroleum ether extract as (9R,10S)-epoxy-16-heptadecene-4, 6-diyne-3-one, (9R,10S)-epoxyheptadecan-4,6-diyne-3-one and 1-methoxy-(9R,10S)-epoxyheptadecan-4,6-diyne-3-one, which inhibit ACAT activity in a dose-dependent manner with IC50 values of 35 µM, 47 µM, and 21 µM, respectively. Additionally, ACAT inhibitors isolated from the hairy roots of P. ginseng, identified as panaxynol, panaxydol, panaxydiol, and panaxytriol, inhibit rat liver ACAT with IC50 values of 94, 80, 45, and 79μM, respectively.
In myocardial ischemia, panaxynol reduces ST-segment elevation by decreasing serum MDA and CTn-I levels, increasing SOD, GSH, and GSH-Px enzyme activities, and enhancing NO concentration and NOS activity, thereby mitigating oxidative damage and myocardial injury (Alanko et al., 1994). GVO has been less well studied in cardiovascular disease, and limited data are available for reference. However, the pharmacological effects of panaxynol in ameliorating myocardial injury have also been demonstrated in studies in other plants. Inflammatory vesicle protein complex (NLRP3) is involved in innate immunity in ischemic heart disease (Wang et al., 2020). Panaxynol also inhibits the NLRP3 inflammasome via the HMGB1/TLR4/NF-κB axis, significantly reducing myocardial infarction area and apoptosis, and alleviating myocardial damage and neutrophil infiltration (Ding et al., 2023).
4.2 Anti-inflammatory effectRelevant pharmacological studies have proved that GVO has anti-inflammatory effects, as shown in Table 3. The main anti-inflammatory metabolite, panaxynol, can non-competitively inhibit 15-hydroxyprostaglandin dehydrogenase in the cytoplasm (Fujimoto et al., 1998). Zuo Xu conducted a study using xylene to induce mouse ear swelling. After treatment with GVO, the earpieces of different experimental groups of mice were weighed and their swelling and inhibition rates were calculated. The results indicated a significant inhibitory effect of GVO on ear swelling in mice. In vitro inflammatory cell experiments showed that GVO possesses the ability to suppress the expression of MyD88 and TLR4 proteins, decrease the phosphorylation level of P65 in RAW264.7 cells, and inhibit the NF-kB signaling pathway, thereby exerting anti-inflammatory effects (Zuo, 2021). Acute lung injury, an acute inflammatory disease, can also be ameliorated by panaxydol (PX), a metabolite of GVO. PX has been shown to significantly improve pathological changes in the lungs of mice, reduce pulmonary edema, inflammation, and ferroptosis (Li et al., 2021). The mechanism involves the selective inhibition and upregulation of the Keap1-Nrf2/HO-1 pathway, which markedly attenuates LPS-induced inflammation and ferroptosis.
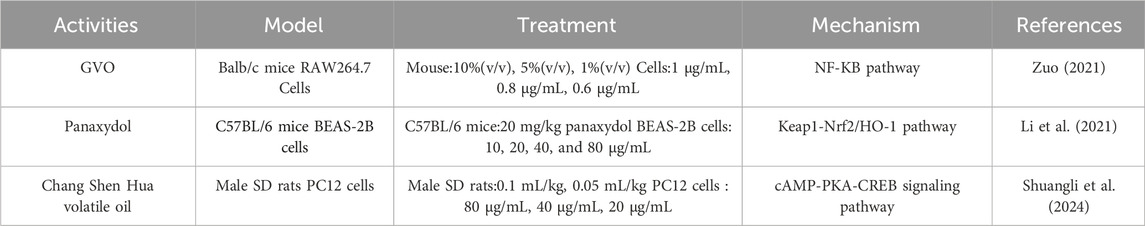
Table 3. The targets and mechanisms of GVO in anti-inflammatory.
Depression, often linked to inflammatory factors, is another condition where GVO shows promise. Depressed patients typically exhibit elevated levels of cytokines such as IL-1β and TNF-α (Rethorst et al., 2013). A novel herbal inhalation preparation combining GVO with other essential oils, known as CSHVO, has been developed. CSHVO has been shown to enhance the proliferation and viability of PC12 cells by inhibiting cort-induced apoptosis. Additionally, CSHVO intervention significantly reduced the expression levels of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and IFN-γ, while increasing the levels of anti-inflammatory cytokines IL-4 and IL-10. This suggests that the anti-depressive activity of CSHVO may be related to the inhibition of inflammatory cytokine release and the alleviation of neuroinflammation (Shuangli et al., 2024). Moreover, panaxynol from other plants has been shown to exert anti-inflammatory effects by inhibiting the secretion of inflammatory cytokines TNF and IL-6 in BV-2 microglial cells, preventing their overactivation. This is achieved through the suppression of the NF-κB/i-κB-α inflammatory signaling pathway, which increases the secretion of brain-derived neurotrophic factor (BDNF) and tyrosine kinase receptor B (TrkB) proteins in the hippocampus of mice, thereby achieving anti-depressive effects (Zhao et al., 2021).
In summary, the anti-inflammatory effects of GVO are mediated through multiple mechanisms, including the inhibition of key inflammatory pathways and cytokines, as well as the modulation of neuroinflammatory responses. These findings highlight the potential of GVO as a therapeutic agent for inflammatory diseases and related conditions. Panaxydol in P. ginseng volatile oil has anti-inflammatory effects. Panaxynol from other plants has shown anti-inflammatory effects, and it remains to be investigated whether it has the same effect in GVO.
4.3 Antibacterial effectsPharmacological studies have shown that the antibacterial mechanism of GVO may involve the synergistic action of multiple metabolites, such as disrupting bacterial cell wall and membrane permeability, affecting bacterial energy metabolism, and inhibiting protein and nucleic acid synthesis (Chen and Fan, 2016). The volatile oil present in the outer cork layer and phloem of P. ginseng roots has inhibitory effects on the growth of various Gram-positive bacteria, including Staphylococcus, Streptococcus, Diphtheria, Listeria, and Streptococcus.
Panaxynol exhibits good antibacterial activity against Staphylococcus aureus, Mycobacterium tuberculosis, Bacillus subtilis, Gram-negative bacteria, and Escherichia coli (Bae et al., 2001). Gram-negative bacteria are known to cause inflammation in the lungs, and lipopolysaccharide (LPS), the principal metabolite of the outer membrane of gram-negative bacteria, is considered one of the major causes of lung diseases (Kolomaznik et al., 2017). Panaxydol can inhibit the activity of LPS, thereby effectively inhibiting gram-negative bacteria. Helicobacter pylori (HP) is recognized a risk factor for gastric cancer and plays a crucial part in the development of gastritis and peptic ulcers (Warren and Marshall, 1983). Pathogenic HP produces urease, an enzyme that breaks down urea into ammonia and carbamate. Research has confirmed that panaxytriol can achieve anti-Helicobacter pylori effects by inhibiting gastric urease and gastric H+/K + ATPase (Bae et al., 2001) (Table 4).
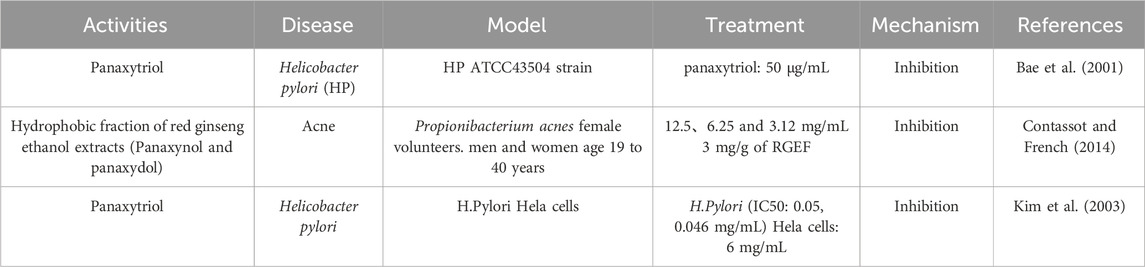
Table 4. The targets and mechanisms of GVO in antibacterial.
Moreover, polyacetylene also has good antifungal (Xie et al., 2022) and antibacterial effects (Kim et al., 2003). Acne is a chronic inflammatory skin disease caused by excessive sebum secretion, proliferation of Propionibacterium acnes, and inflammatory response (Contassot and French, 2014). Panaxynol and panaxydol have shown selective inhibitory effects on P. acnes, making them effective in treating acne (Hou et al., 2019).
4.4 Anticancer effectsAmong the pharmacological effects of GVO, its anticancer activity has been extensively studied. The anticancer activity of P. ginseng is primarily attributed to its lipophilic metabolites. Hexane extraction maximizes the recovery of anticancer active metabolites, demonstrating strong in vitro inhibitory effects on the proliferation of human liver and breast cancer cells in a concentration-dependent manner (Lee et al., 2009). Polyacetylenes such as panaxynol, panaxydol, and panaxytriol are considered the main anticancer metabolites, exhibiting antiproliferative effects on mouse sarcoma, leukemia, human colon cancer, and human ileocecal adenocarcinoma cell lines. Panaxydol accelerates cell cycle progression from G1 to S phase by reducing Cdk1 activity and upregulating p27KIP1 protein expression, inhibiting human renal cell carcinoma proliferation (Sohn et al., 1998; Moon et al., 2000). Panaxydol induces cancer cell apoptosis by inhibiting EGFR activation and endoplasmic reticulum stress, suppressing tumor growth in mice (Figure 9) (Kim et al., 2011; Kim et al., 2016). Panaxynol significantly reduces MMP-2 mRNA and protein levels in melanoma cells (B16F10) at a concentration of 3 μg/mL, inhibiting cancer cell invasion and migration (Yun et al., 2015) (Table 5).
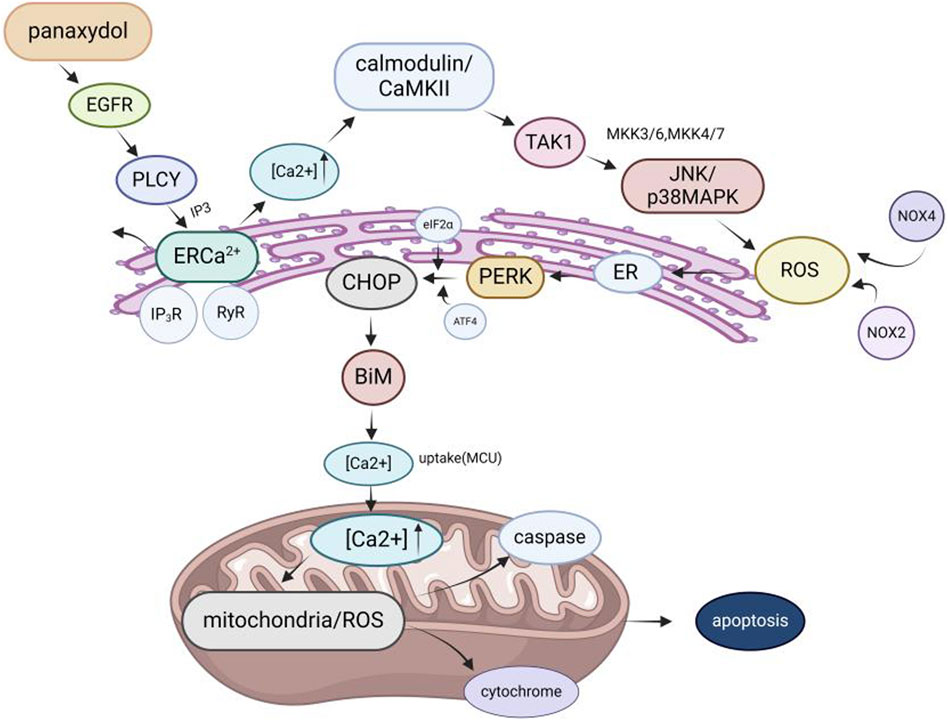
Figure 9. Molecular mechanism of GVO for anti-cancer. The mechanism of panaxydol induced cell apoptosis involves a rapid increase in cytoplasmic Ca2+concentration, with excess Ca2+ transferring from the endoplasmic reticulum (ER) to mitochondria. The release of E [Ca2+] and the resulting increase in Ca2+ activate p38 and JNK, while p38/JNK further activates NADPH oxidase. NADPH oxidase activates and induces oxidative stress, triggering mitochondria-dependent apoptosis.
Comments (0)